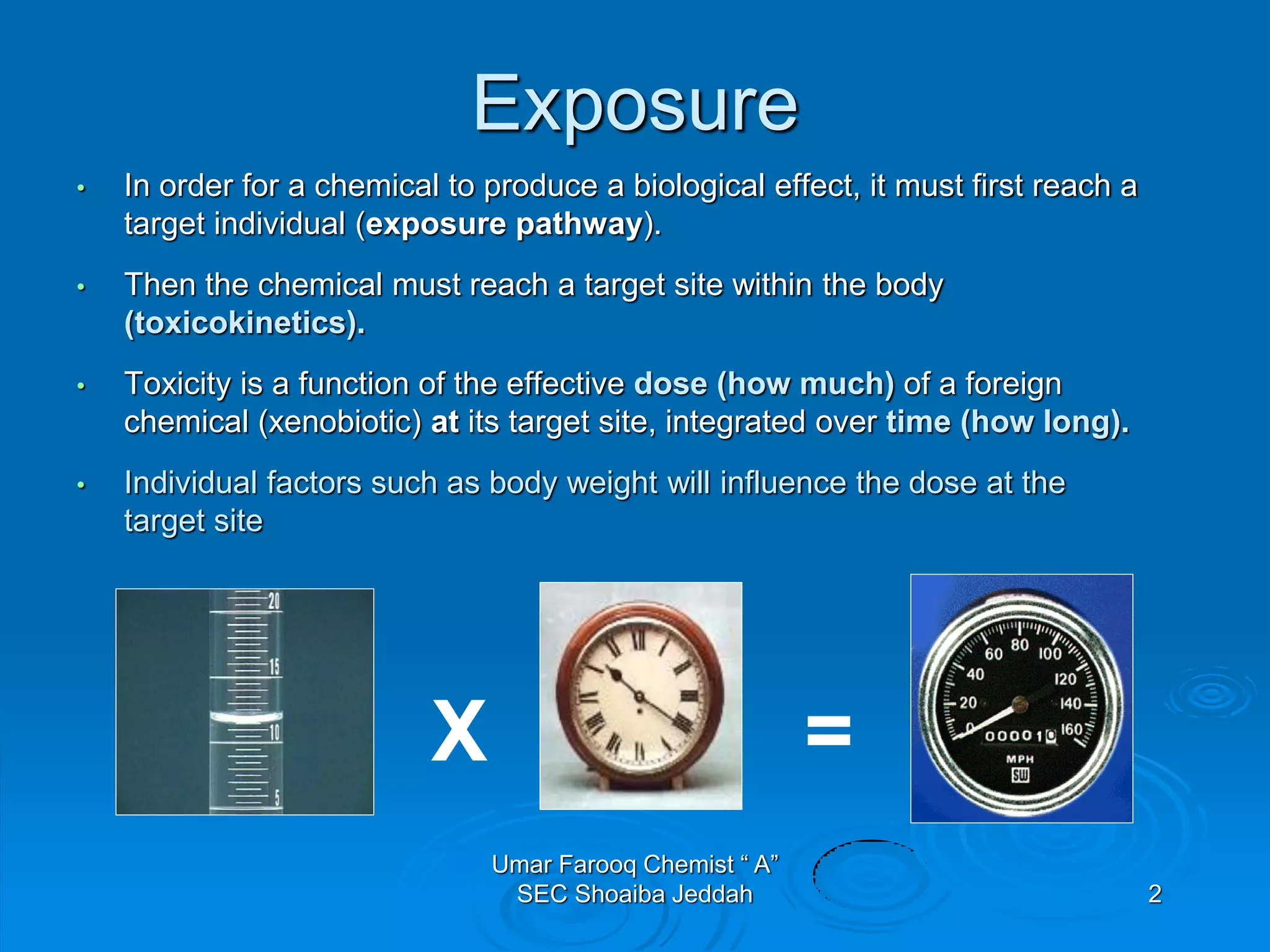
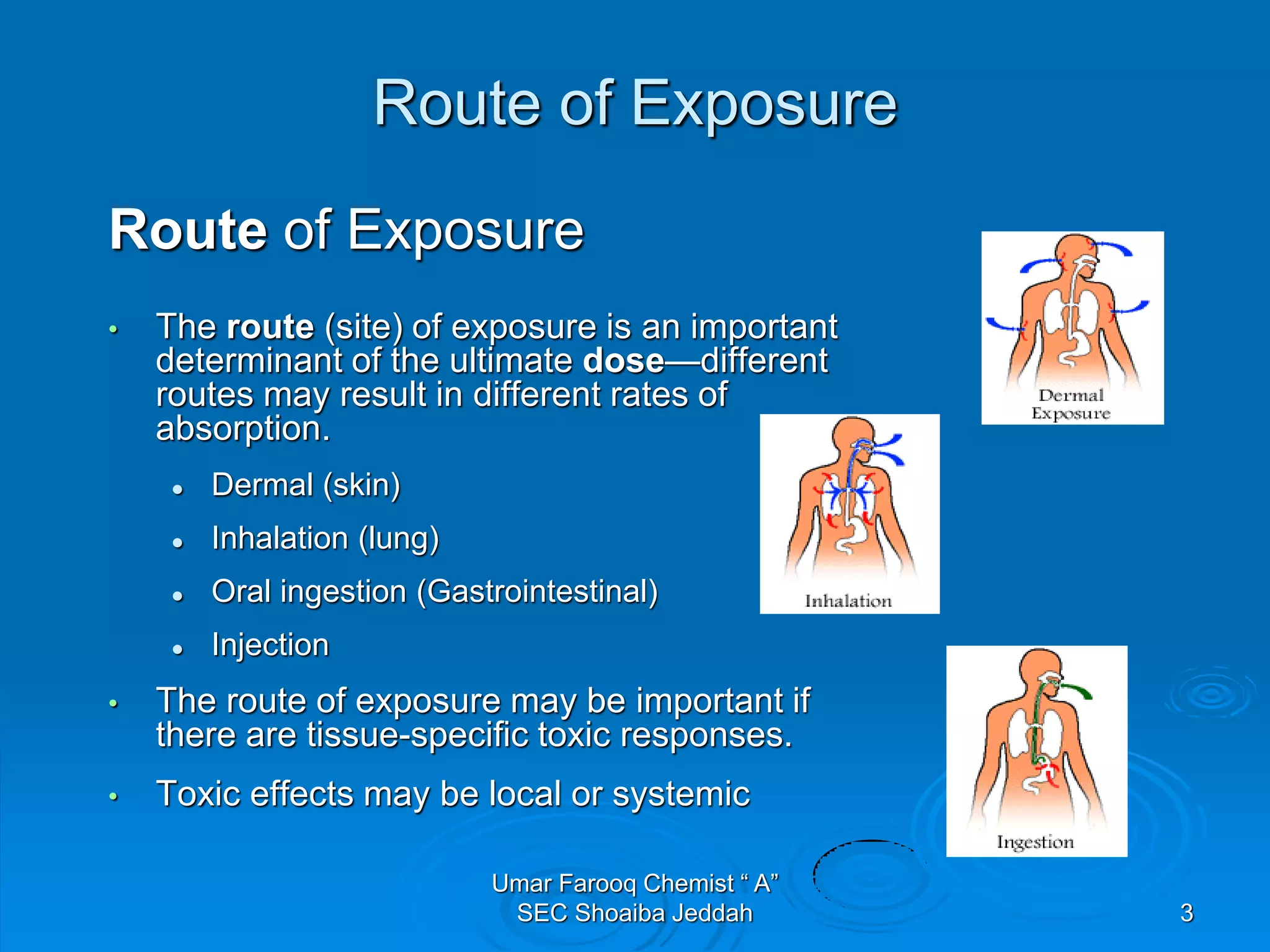
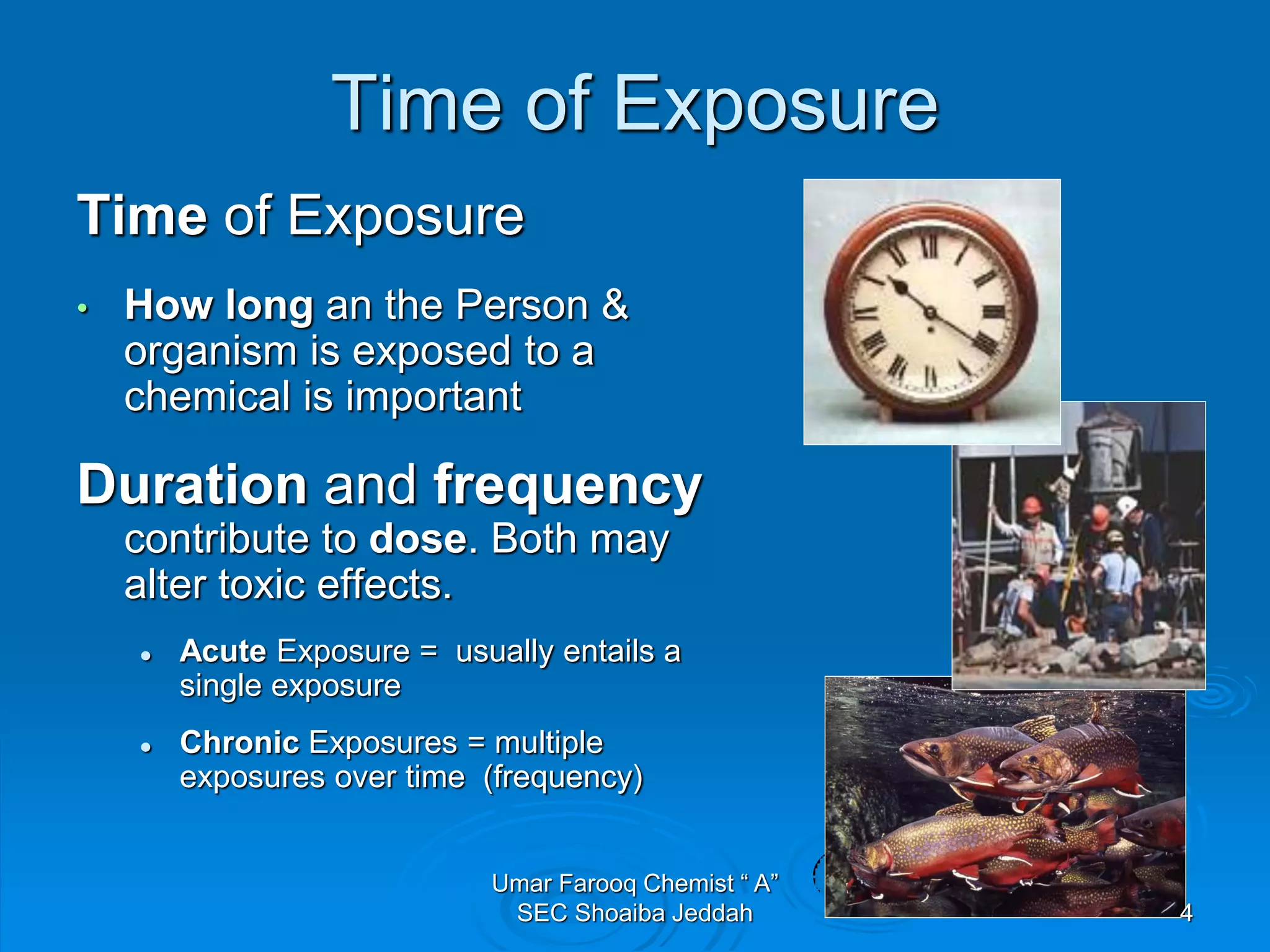
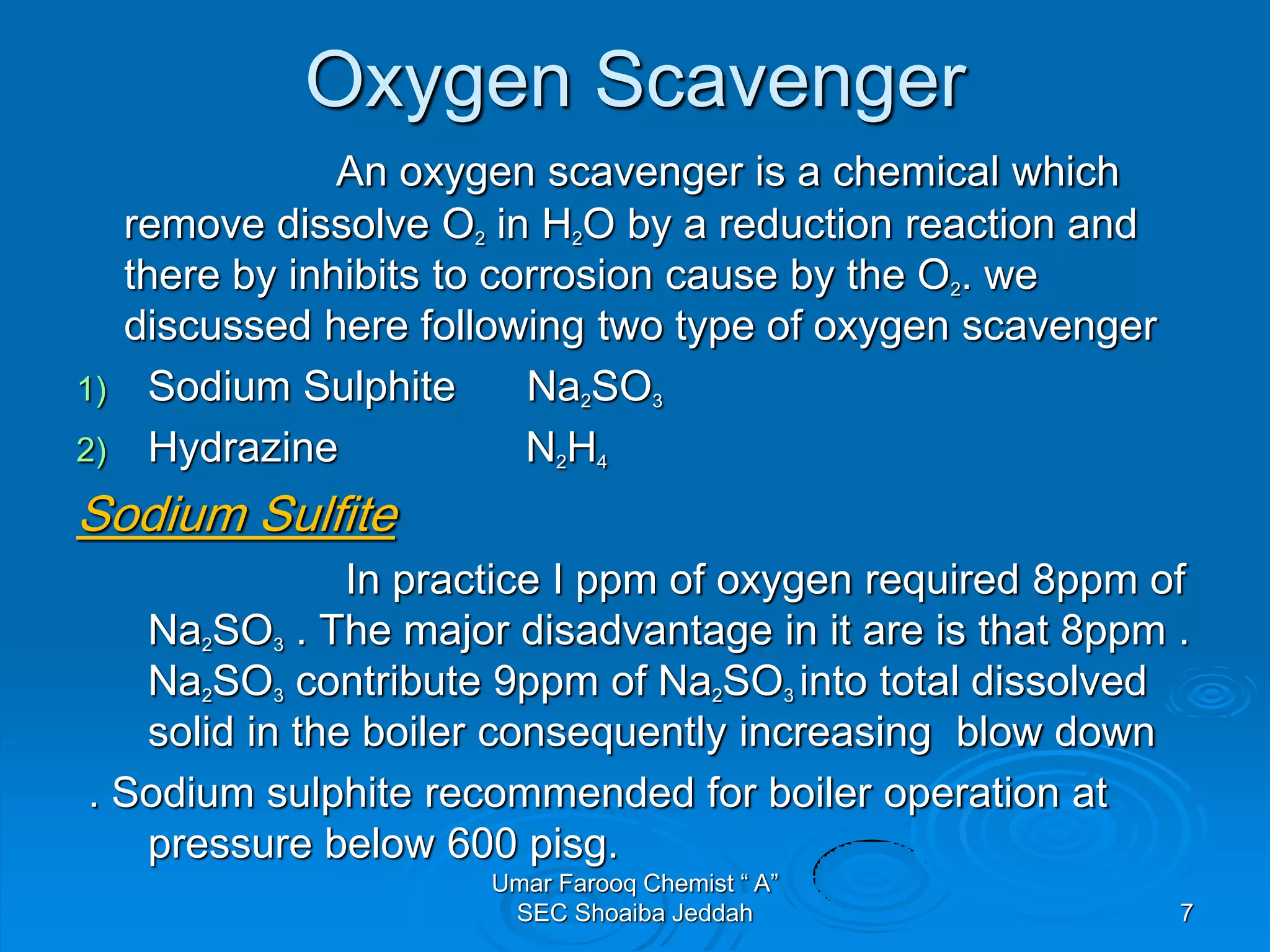
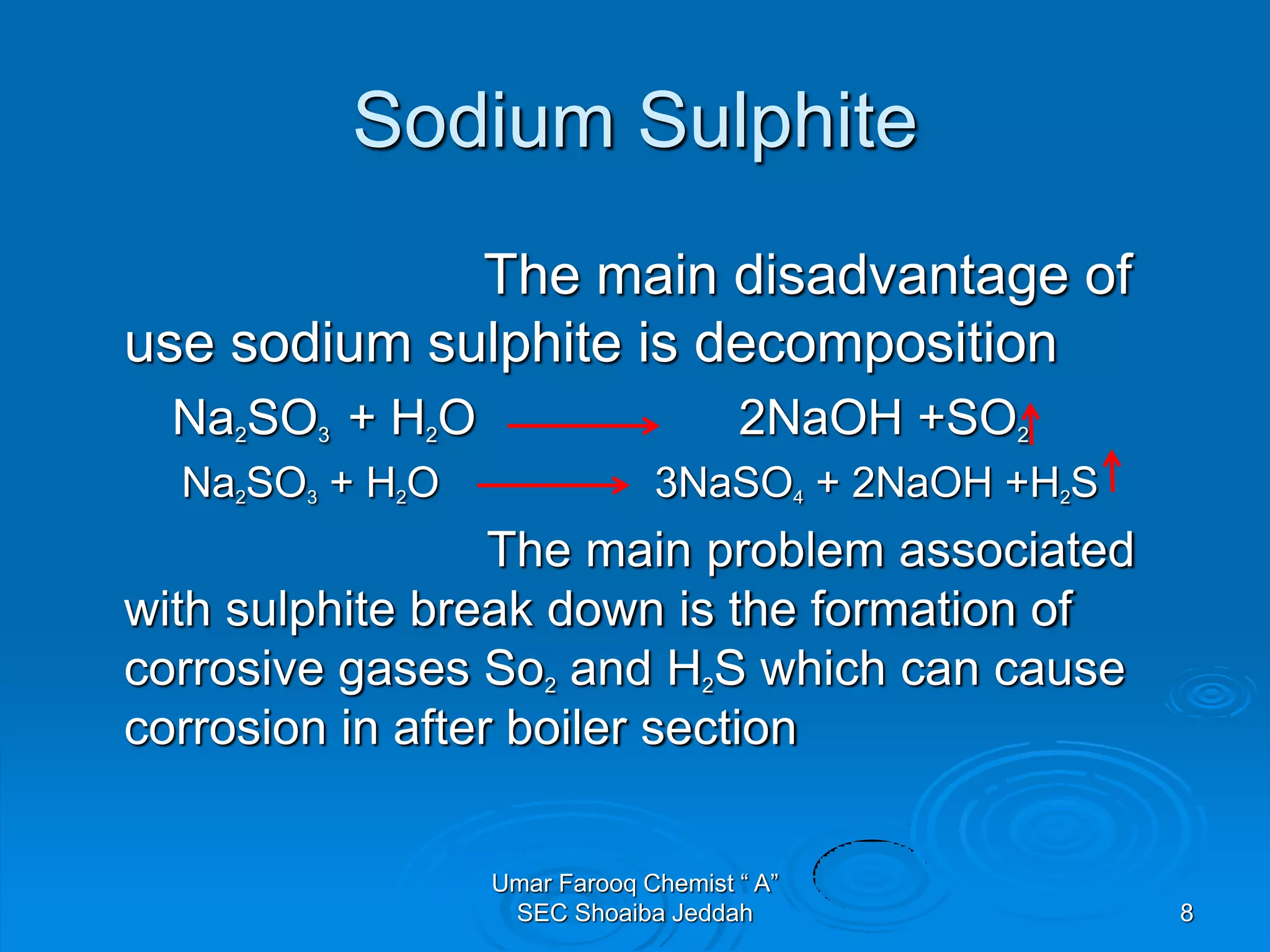
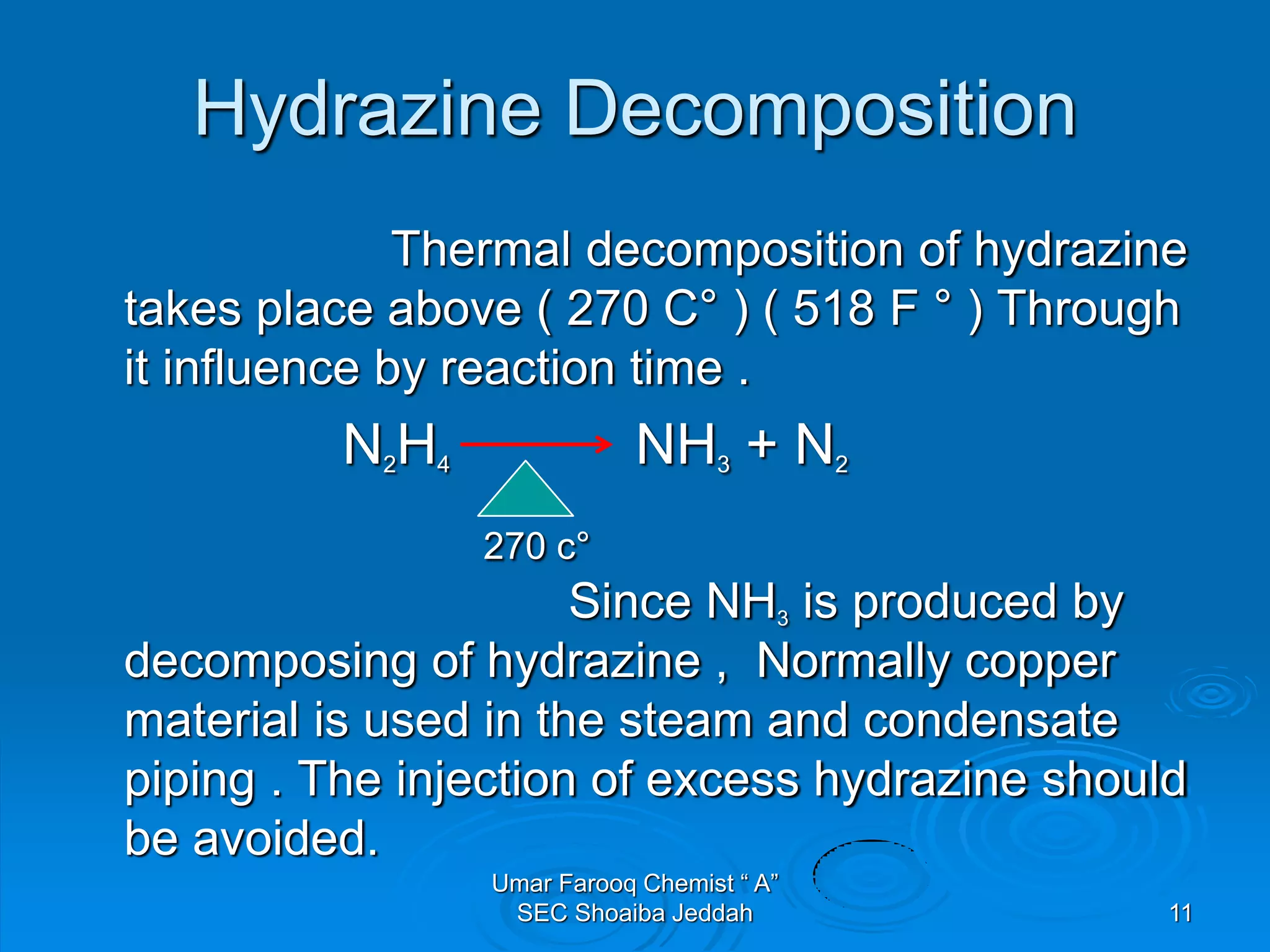
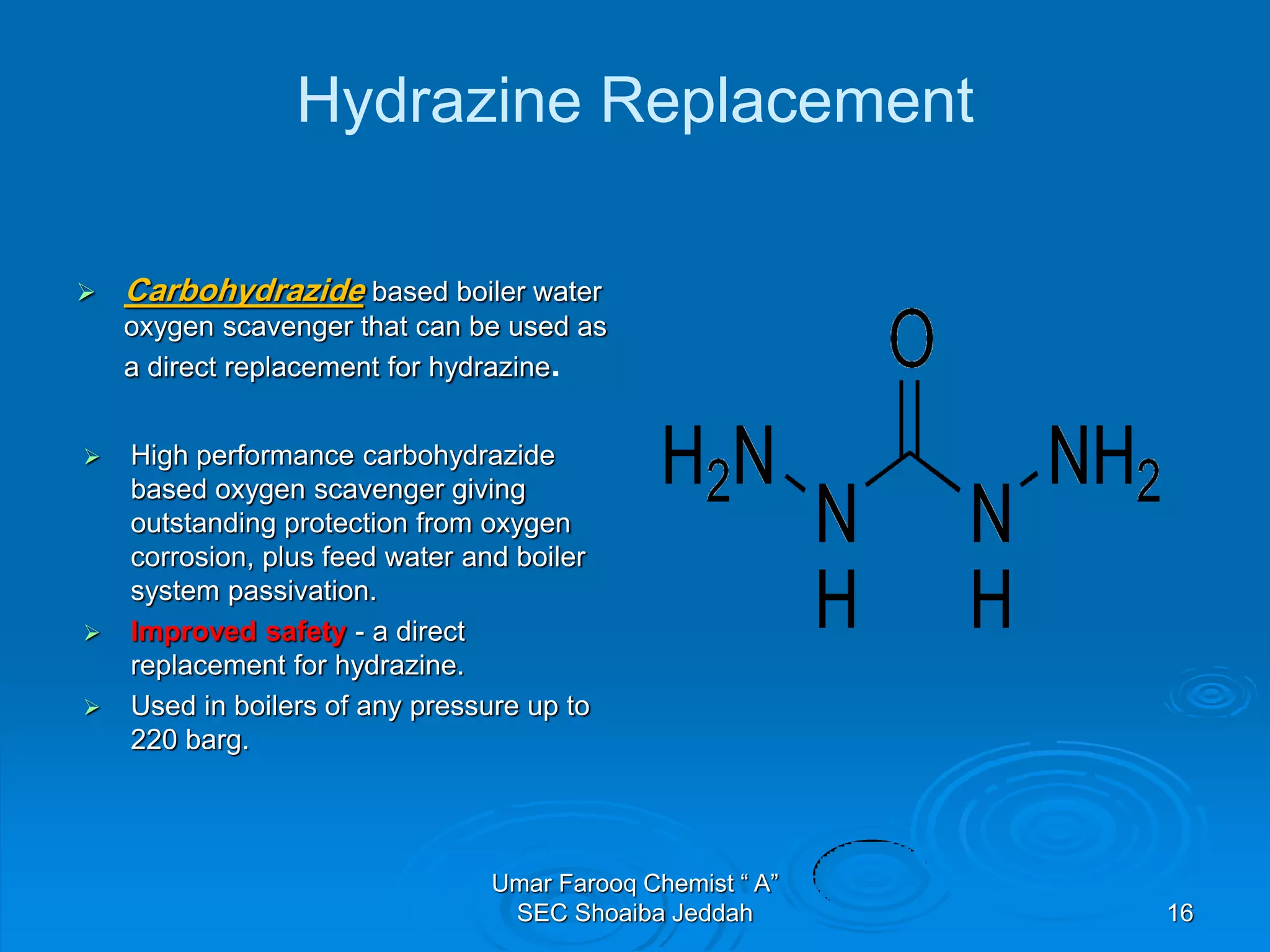
The document discusses oxygen scavengers, their uses, and safety precautions. It describes two common oxygen scavengers - sodium sulfite and hydrazine. Sodium sulfite is effective at removing oxygen below 600 PSIG but contributes to total dissolved solids. Hydrazine can remove small amounts of oxygen without affecting water chemistry. However, hydrazine is highly toxic, flammable, and decomposes above 270°C, producing ammonia. The document outlines safety equipment needed for handling hydrazine due to its toxicity and lists potential carcinogenic and organ-damaging effects. Carbohydrazide is proposed as a safer alternative oxygen scavenger.