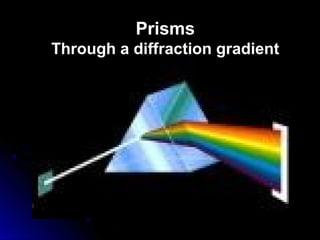
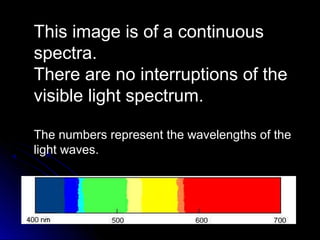
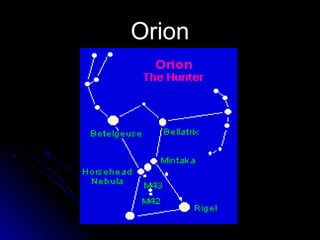
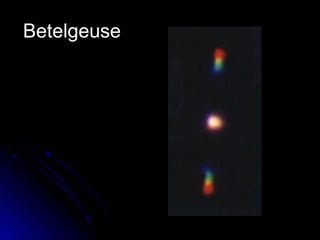
The document discusses spectranomy and how prisms can be used to separate light into a continuous spectrum. It provides examples of bright line spectra for various elements like mercury, hydrogen, nitrogen, argon, and neon that can be used to identify unknown elements. The end discusses the stars Rigel and Betelgeuse in the constellation Orion.