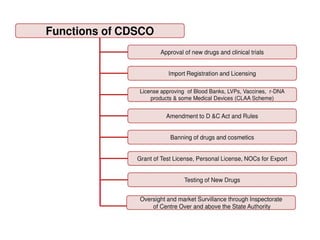
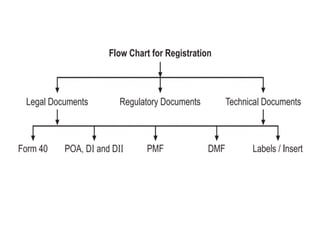
The document outlines the role of the Central Drugs Standard Control Organization (CDSCO) in India, detailing its responsibilities in drug approval, clinical trials, and regulation of drug quality. It also describes the Certificate of Pharmaceutical Product (COPP), which is necessary for drug registration in importing countries, and lists application requirements for obtaining this certificate. The mission of the CDSCO is to safeguard public health by ensuring the safety, efficacy, and quality of pharmaceuticals and medical devices.