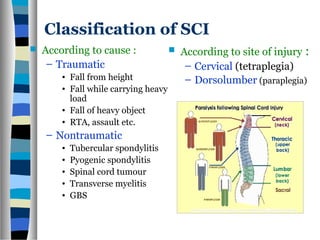
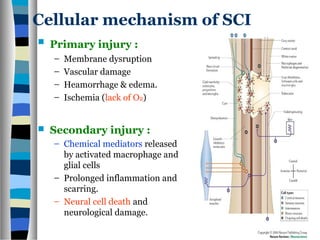
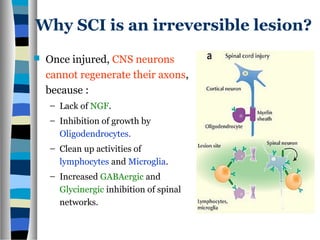
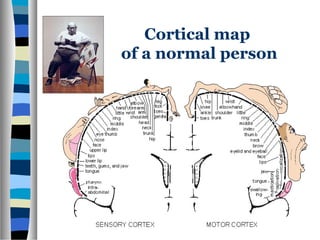
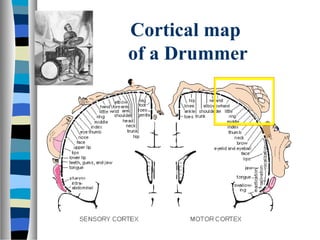
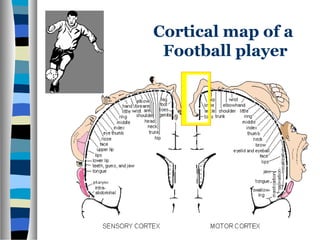
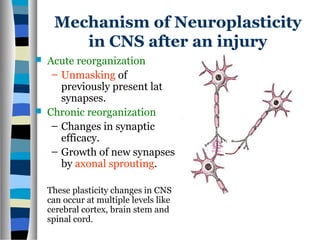
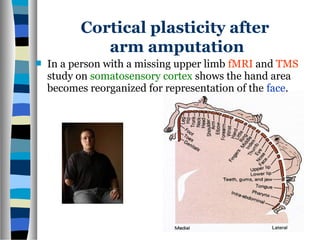
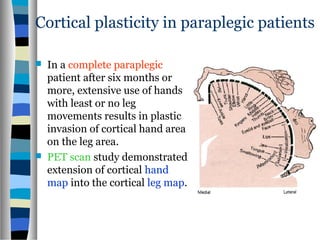
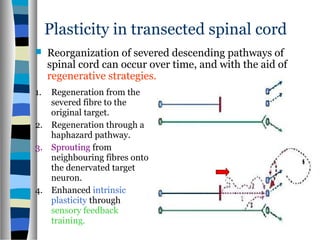
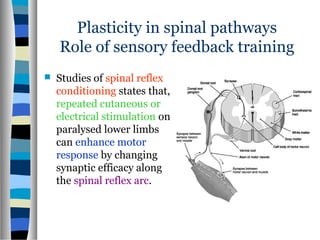
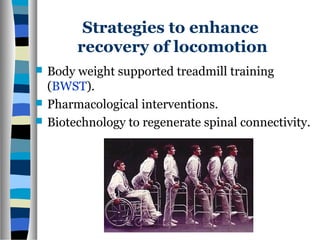
This document discusses neuroplasticity and recovery after spinal cord injury. It classifies spinal cord injuries and explains that neuroplasticity refers to the brain's ability to form new neural connections. It provides examples of cortical reorganization and plasticity in the brain after injuries like amputation or spinal cord injury. The document also discusses how sensory feedback training and treadmill training can enhance neuroplasticity and promote recovery of locomotion through reorganization of spared pathways in the spinal cord.