1) Neonatal seizures have an incidence of 10.3-36.1 per 1000 live births depending on gestational age and birth weight. They can be subtle, clonic, tonic, or myoclonic.
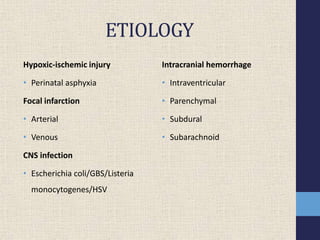
2) The main etiologies of neonatal seizures include hypoxic-ischemic injury, infections, hemorrhage, inborn errors of metabolism, and structural abnormalities.
3) Treatment involves correcting hypoglycemia and hypocalcemia if present, followed by antiepileptic drugs like phenobarbital, phenytoin, or benzodiazepines. Weaning of antiepileptic drugs depends on the neurological exam and EEG findings.