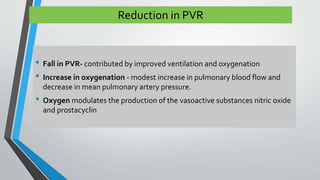
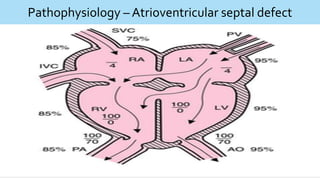
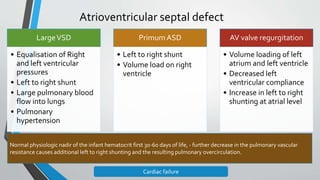
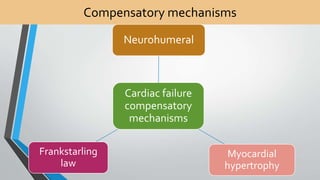
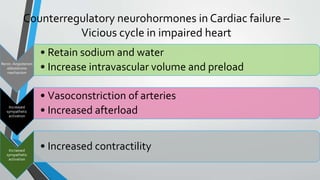
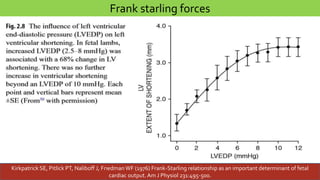
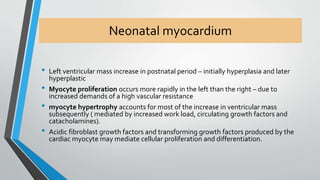
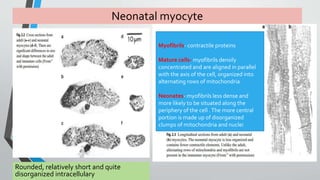
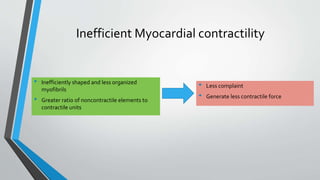
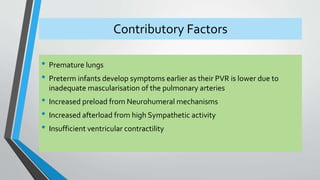
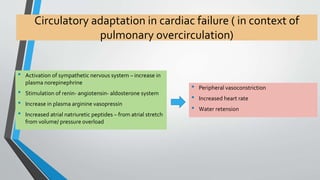
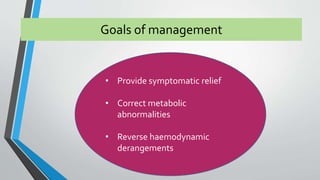
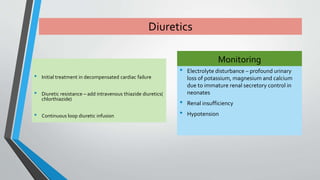
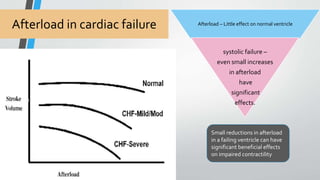
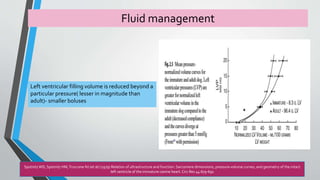
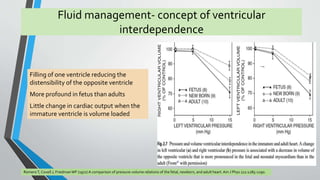
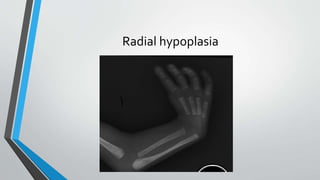
This document discusses neonatal cardiac failure, including the pathophysiology of atrioventricular septal defect. It notes that the neonatal myocardium is anatomically different from the mature heart, with less organized myofibrils and contractile efficiency. This makes the neonatal heart more dependent on compensatory mechanisms like neurohormonal activation and the Frank-Starling response. Medical management aims to reduce afterload and preload on the heart through diuretics and ACE inhibitors while providing respiratory support. Surgical intervention may be needed to correct underlying structural defects.