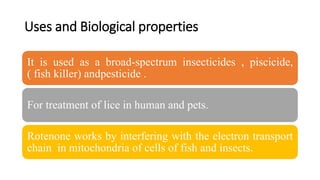
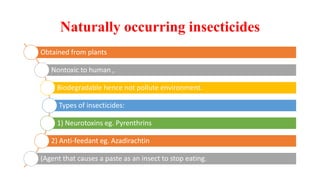
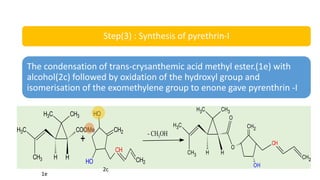
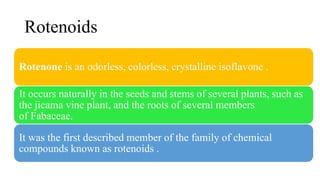
The document discusses naturally occurring insecticides, focusing on pyrethrins and rotenoids, which are derived from plant sources and are biodegradable, making them environmentally friendly. Pyrethrins, obtained from chrysanthemum flowers, act as potent neurotoxins, while rotenoids, derived from plants like the jicama vine, serve as broad-spectrum insecticides but are mildly toxic to humans. The synthesis processes for pyrethrin and the properties of rotenoids are also outlined, highlighting their biological functions and applications.










![Structure of Rotenoids
Rotenoids are naturally occurring substances containing a cis-fused
tetrahydrochromeno[3,4-b]chromene nucleus.[](https://image.slidesharecdn.com/naturalinsecticidesforslideshare-230621174721-24103bd4/85/Natural-insecticides-pptx-14-320.jpg)