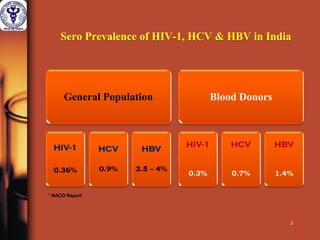
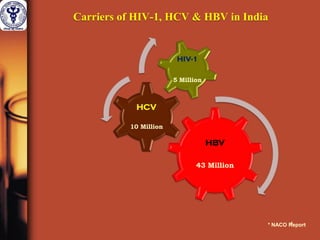
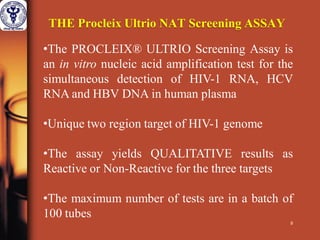
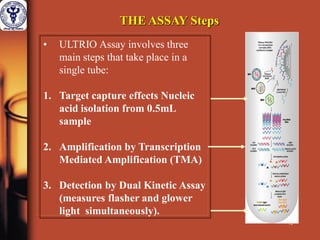
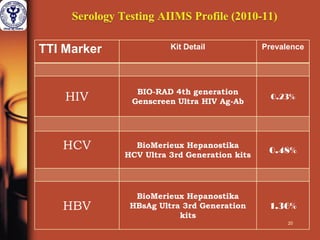
1. The document discusses the use of nucleic acid testing (NAT) to screen blood donations for HIV, HCV, and HBV. NAT helps reduce the window period by directly detecting viral nucleic acids, providing an additional layer of safety beyond serological testing alone.
2. Data from testing over 40,000 blood donations in India showed a NAT yield rate of 1 in 598 donations, detecting 68 donations that were infectious by NAT but non-reactive by serology.
3. Studies comparing different NAT assays found that the Ultrio Plus assay had greater sensitivity than older assays, detecting more true positive donations during the window period.