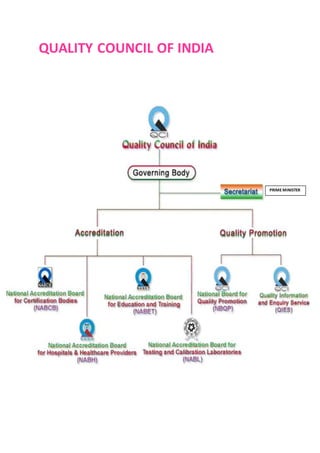
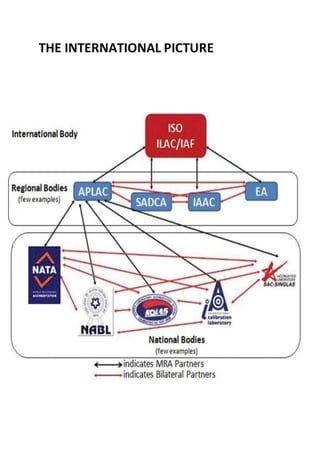
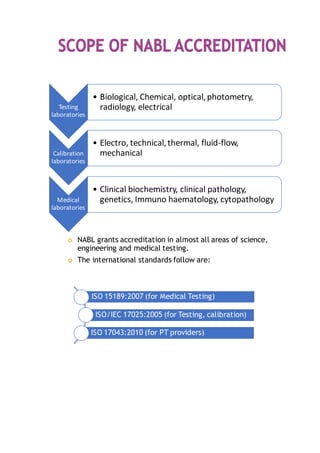
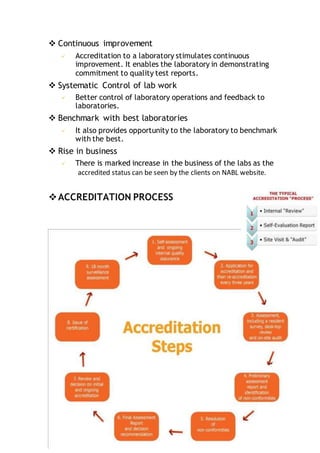
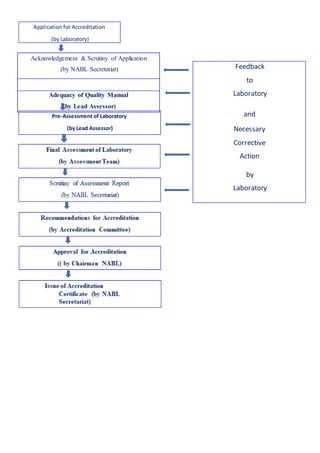
NABL accredits testing and calibration laboratories in India to maintain quality and standards. It is governed by the Quality Council of India (QCI) and operates according to international standards ISO/IEC 17011. NABL accreditation provides benefits such as increased confidence in lab reports, recognition both nationally and internationally, and opportunities for continuous improvement and benchmarking. The accreditation process involves application, pre-assessment of the laboratory, feedback and corrective action, and assessment to determine if the laboratory meets the requirements.