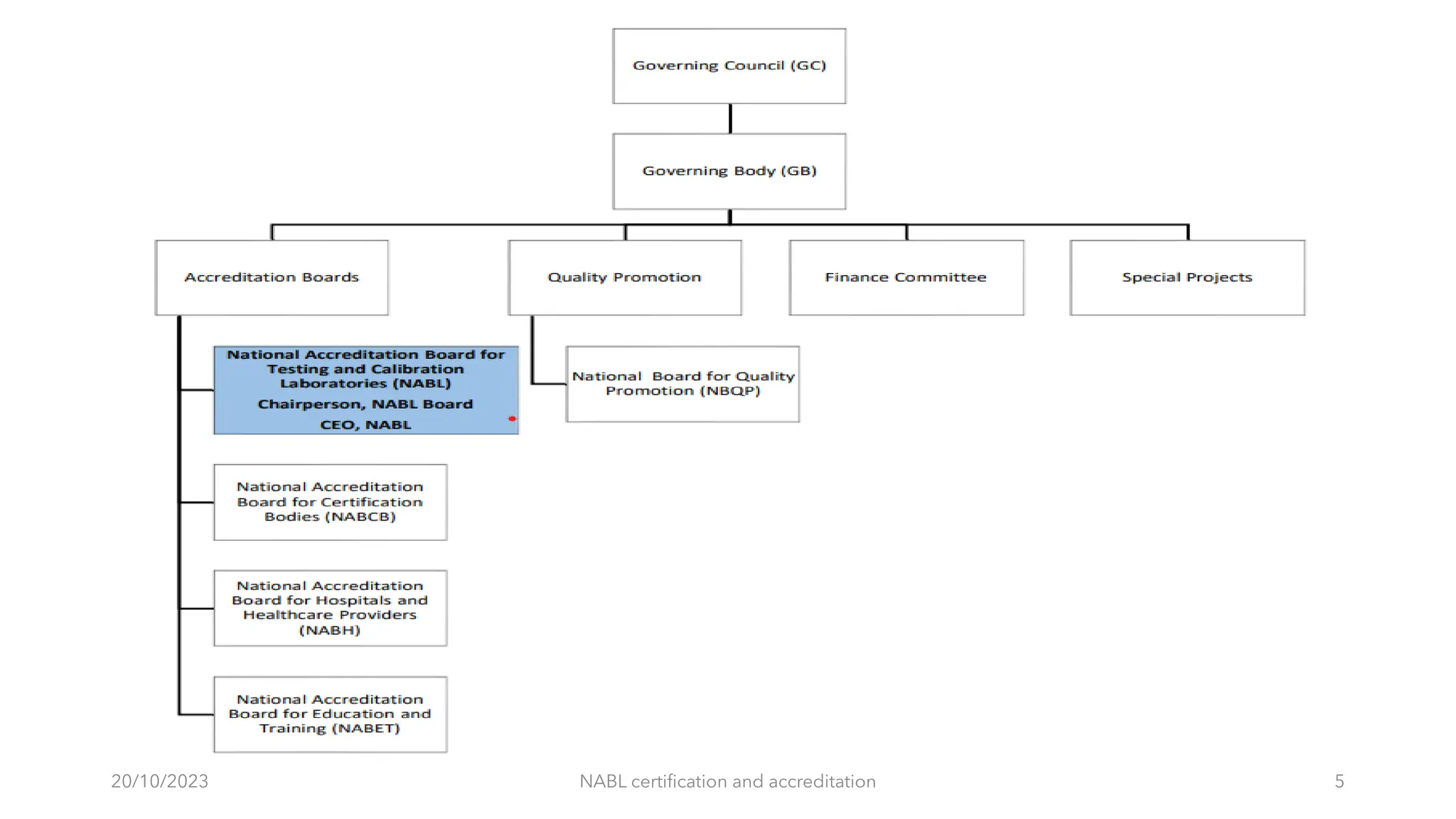
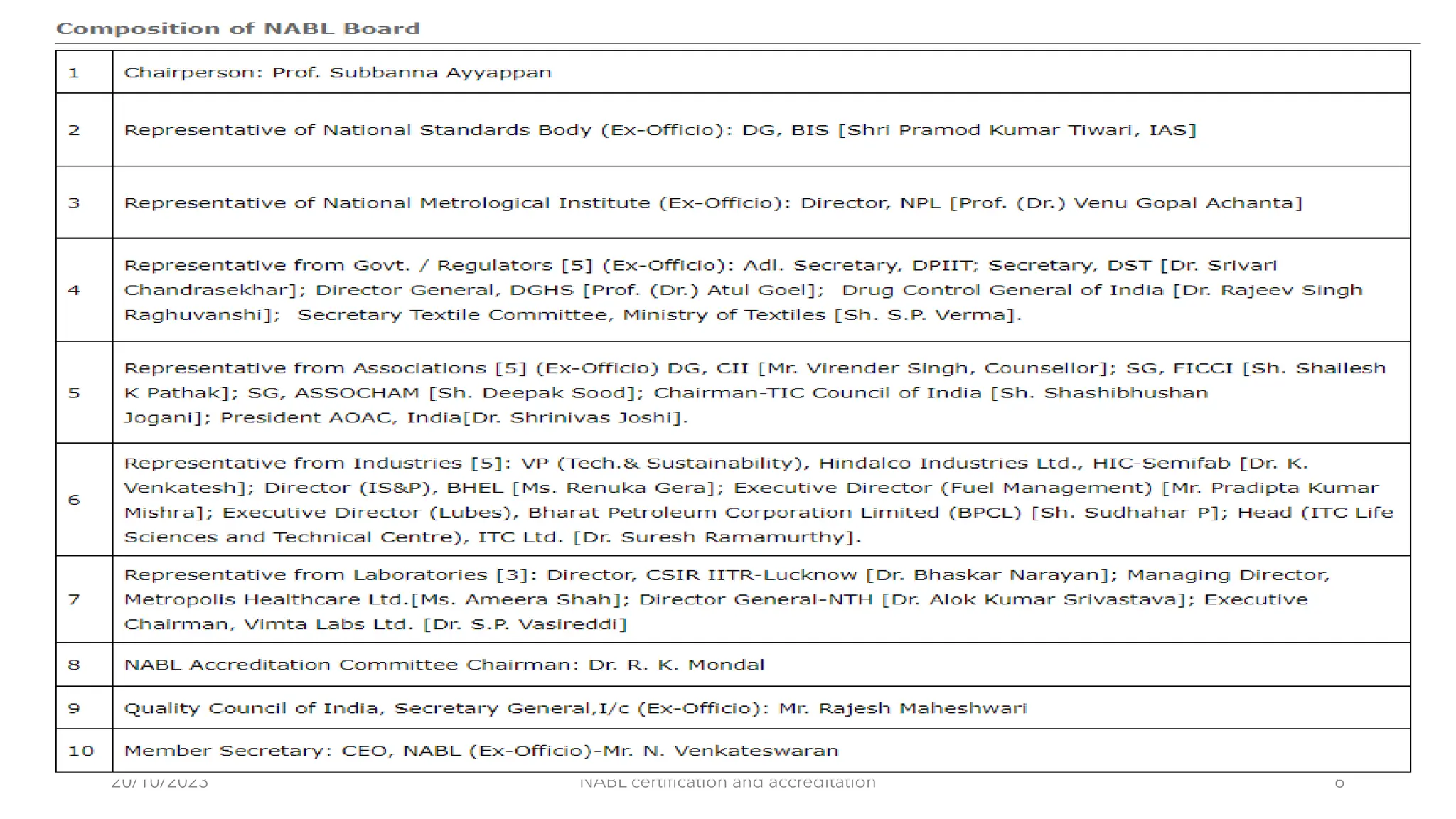
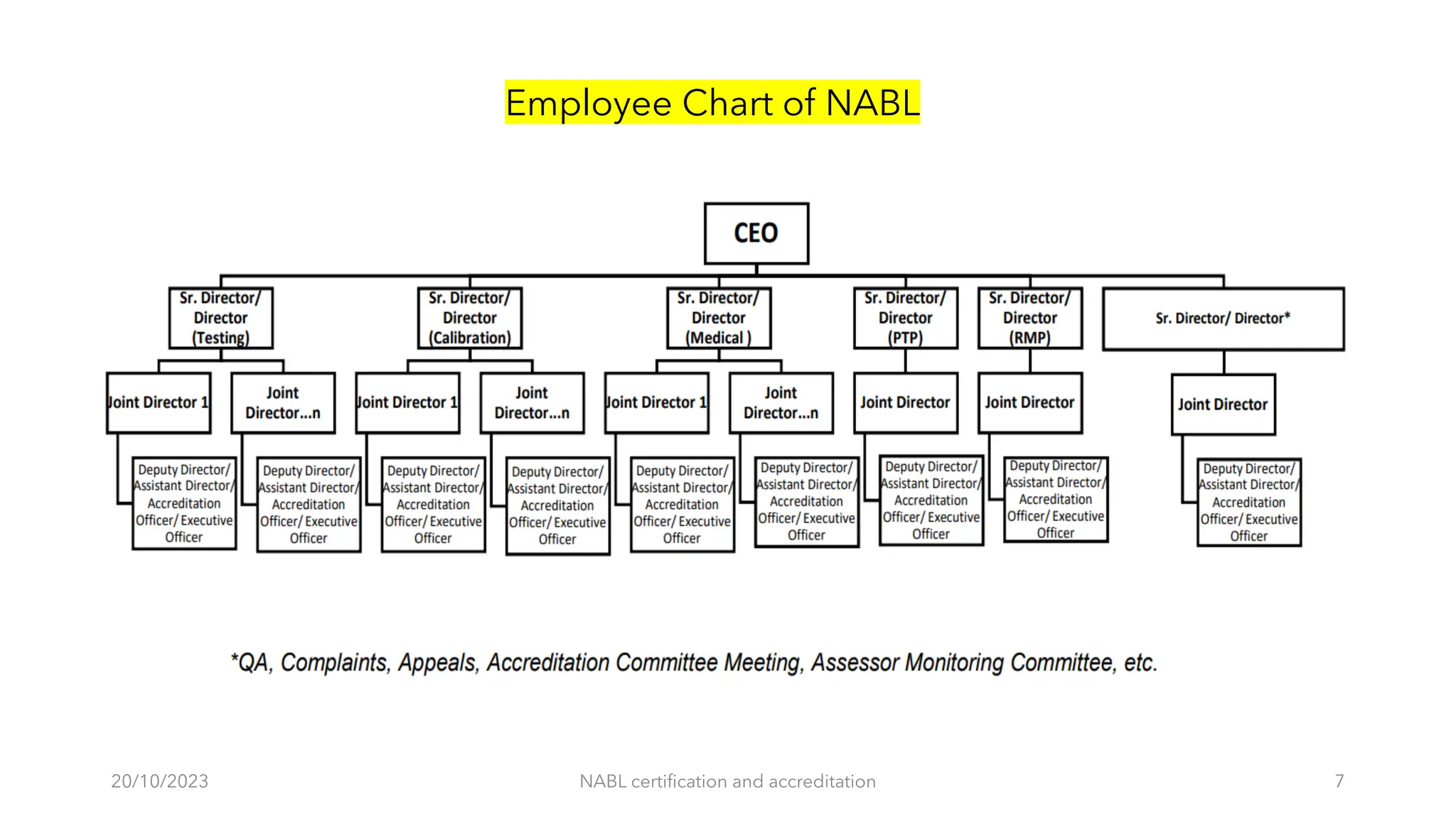
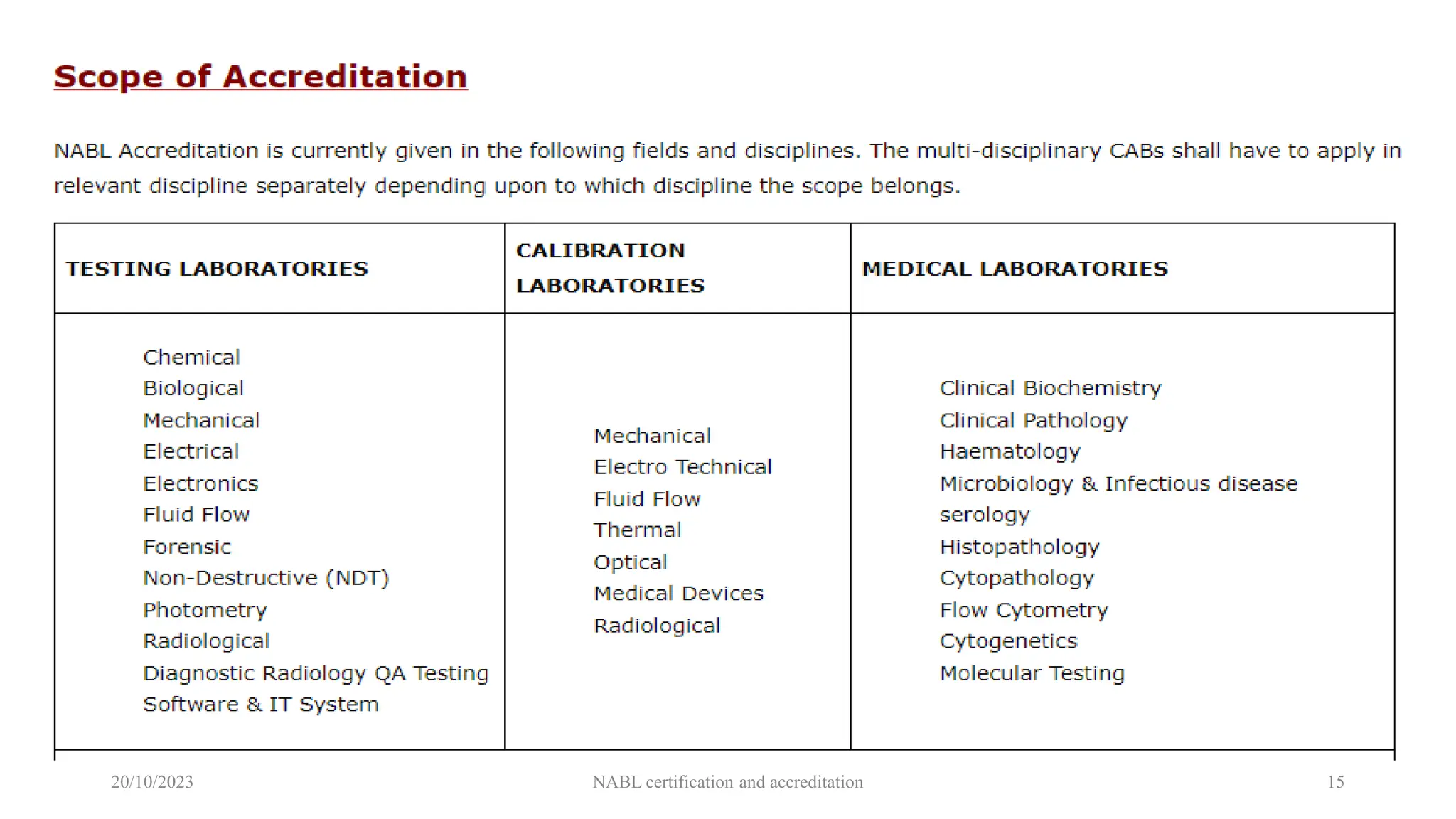
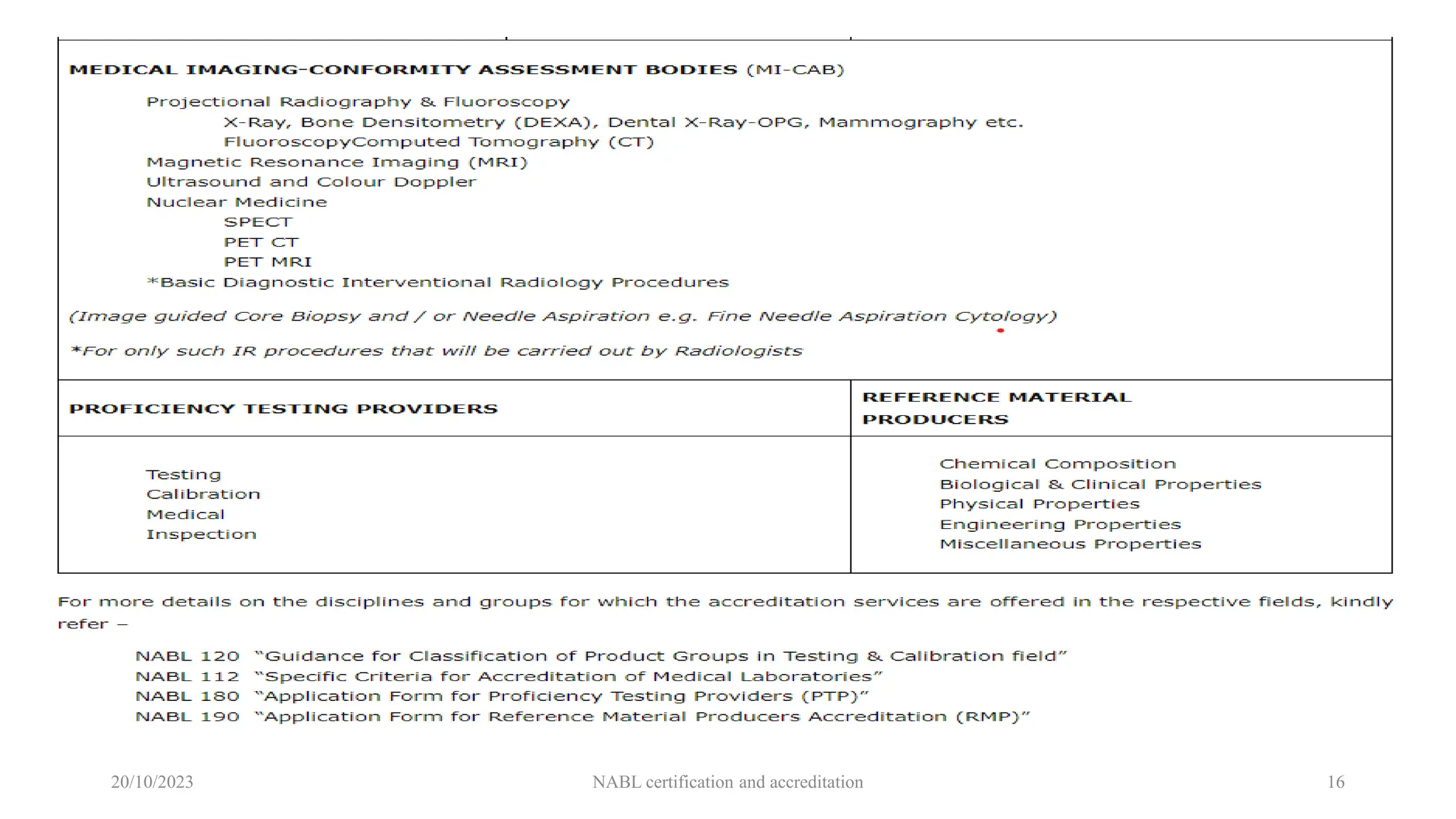
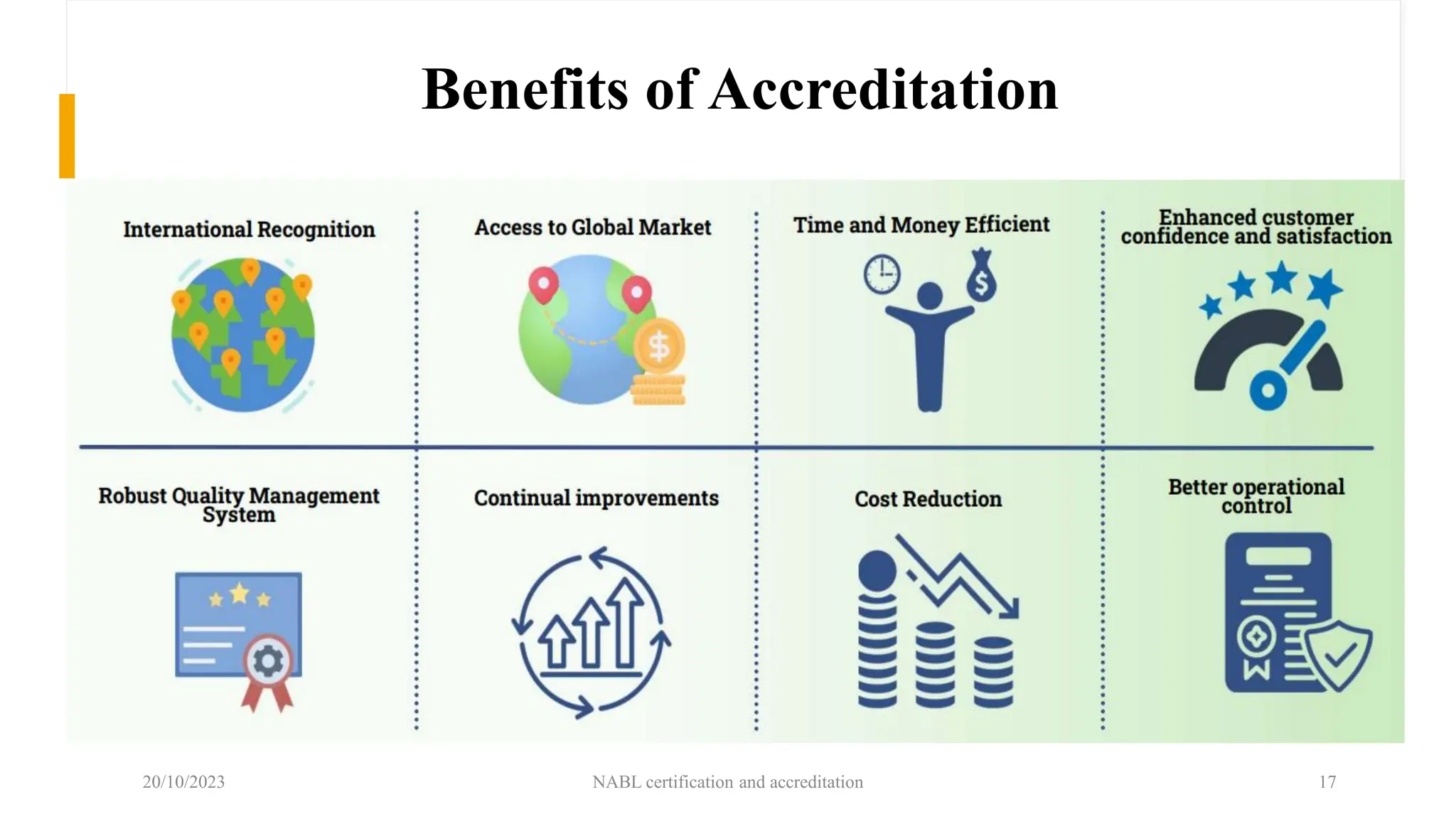
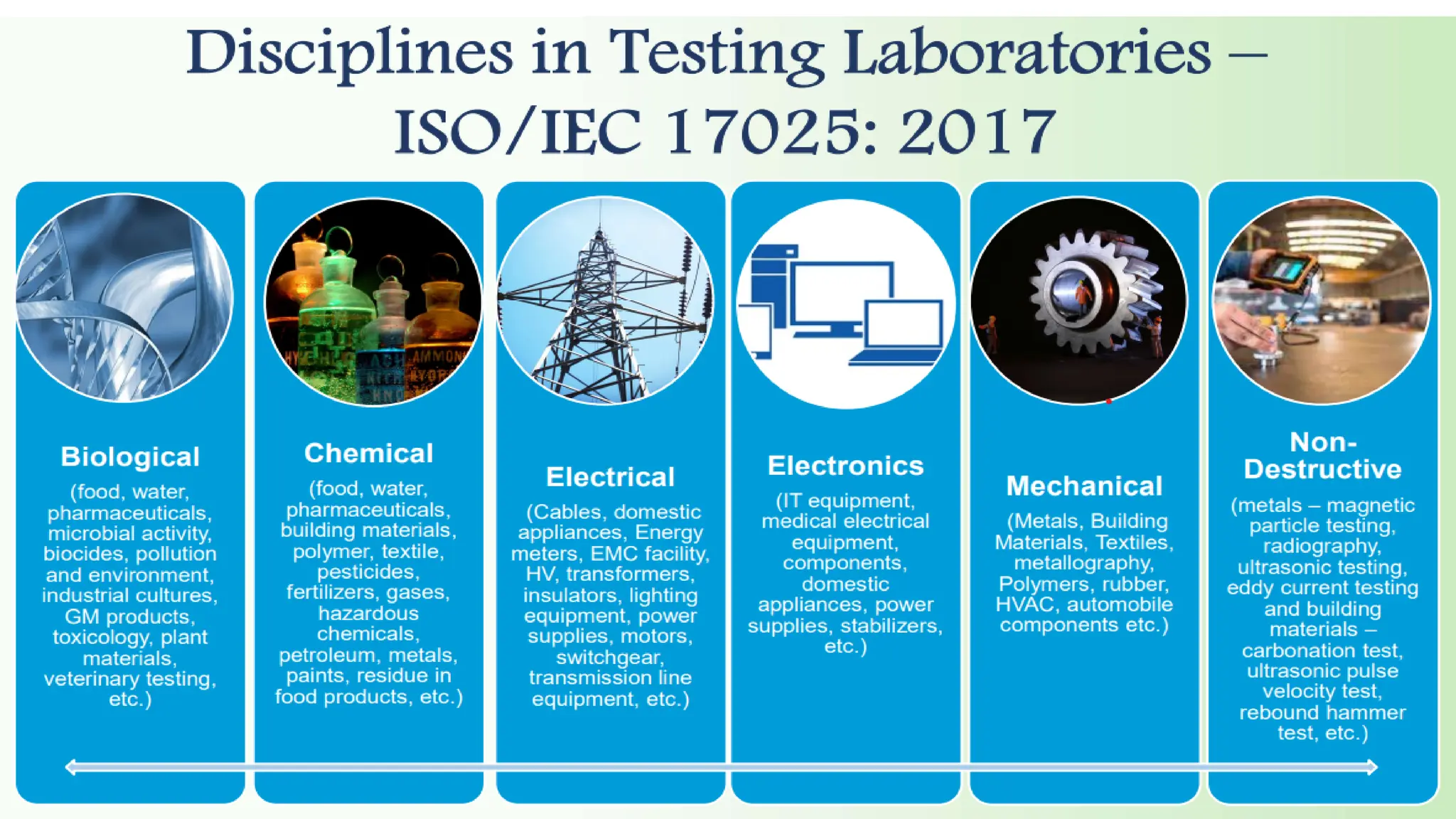
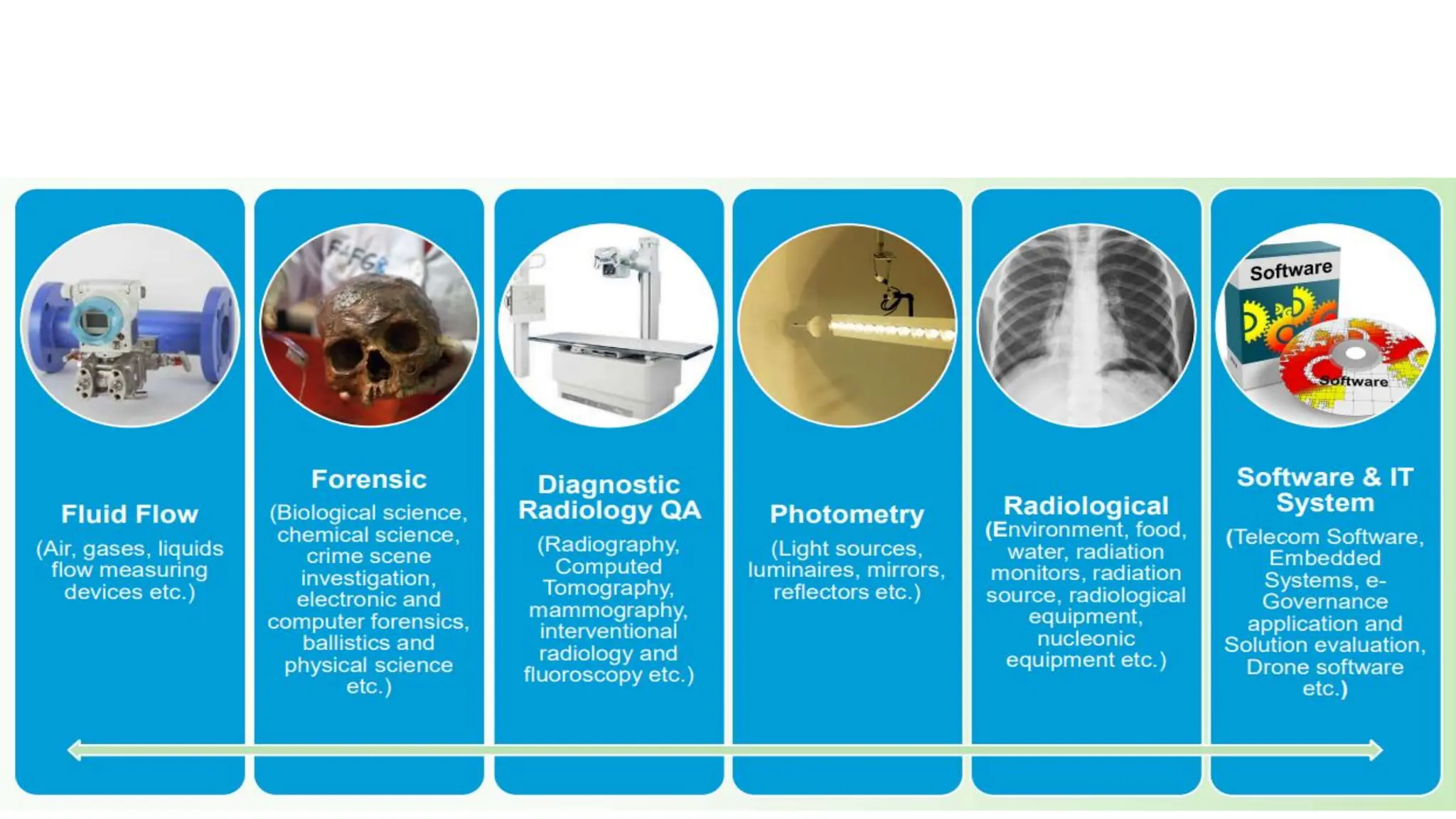
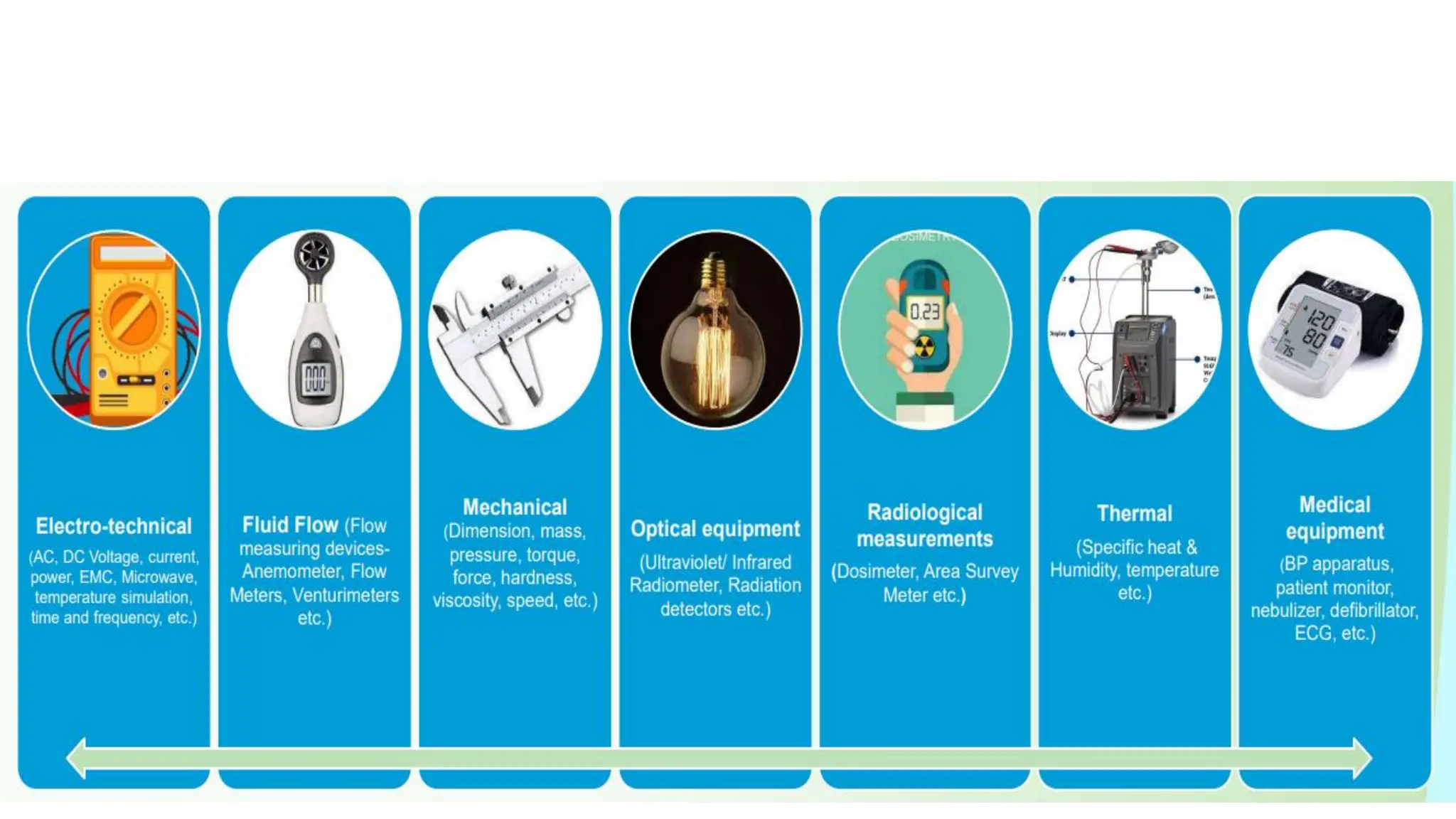
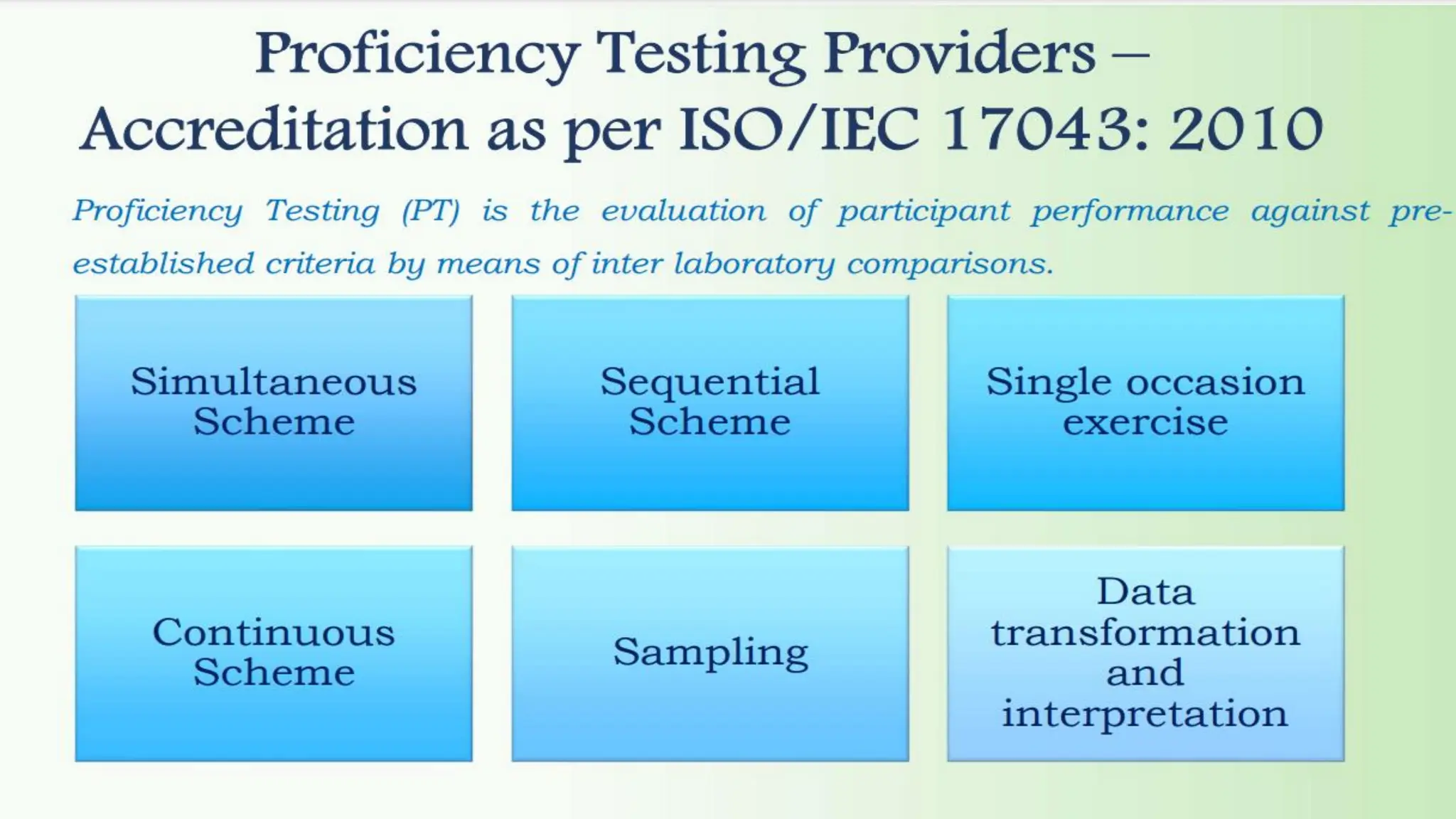
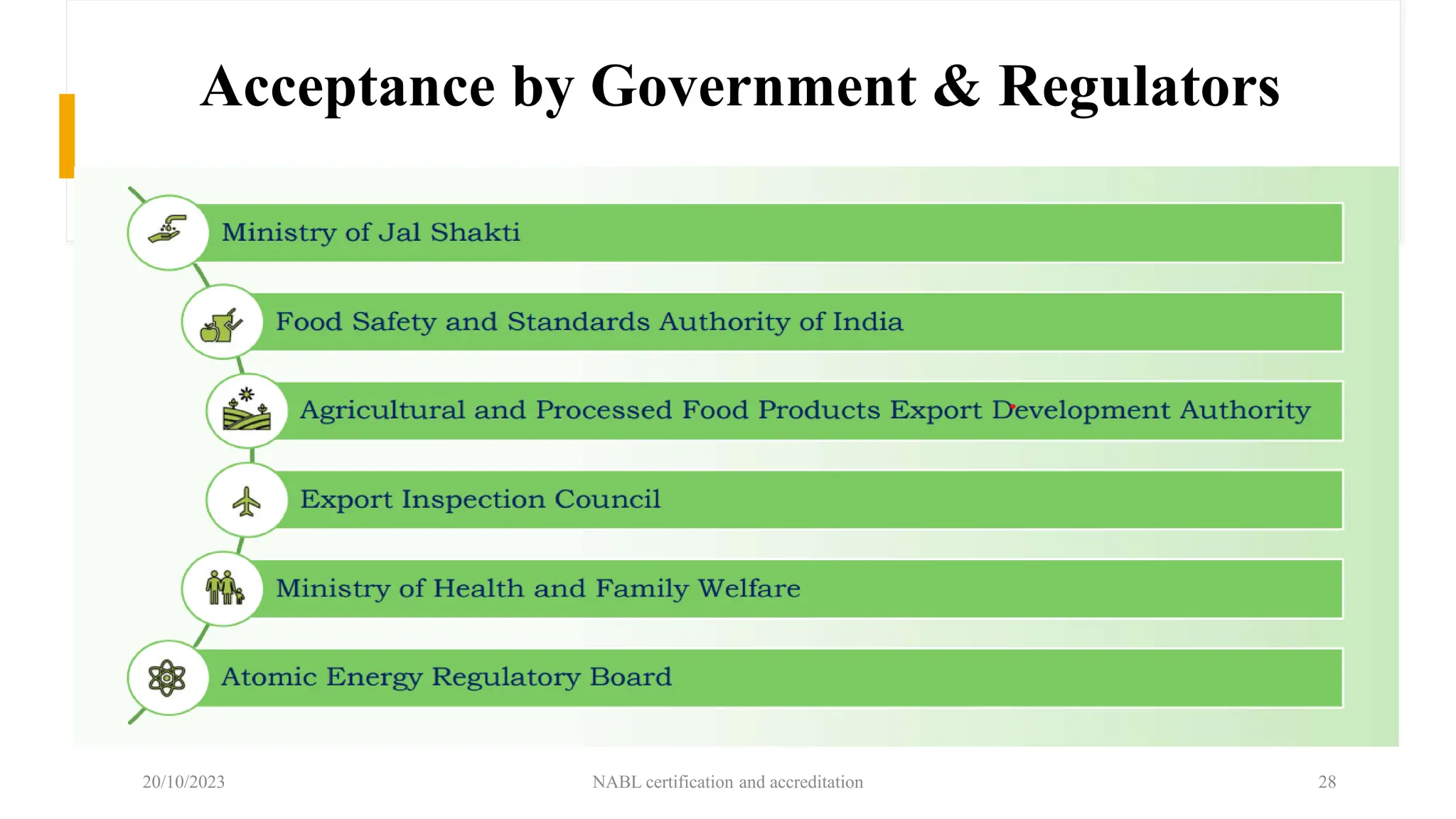
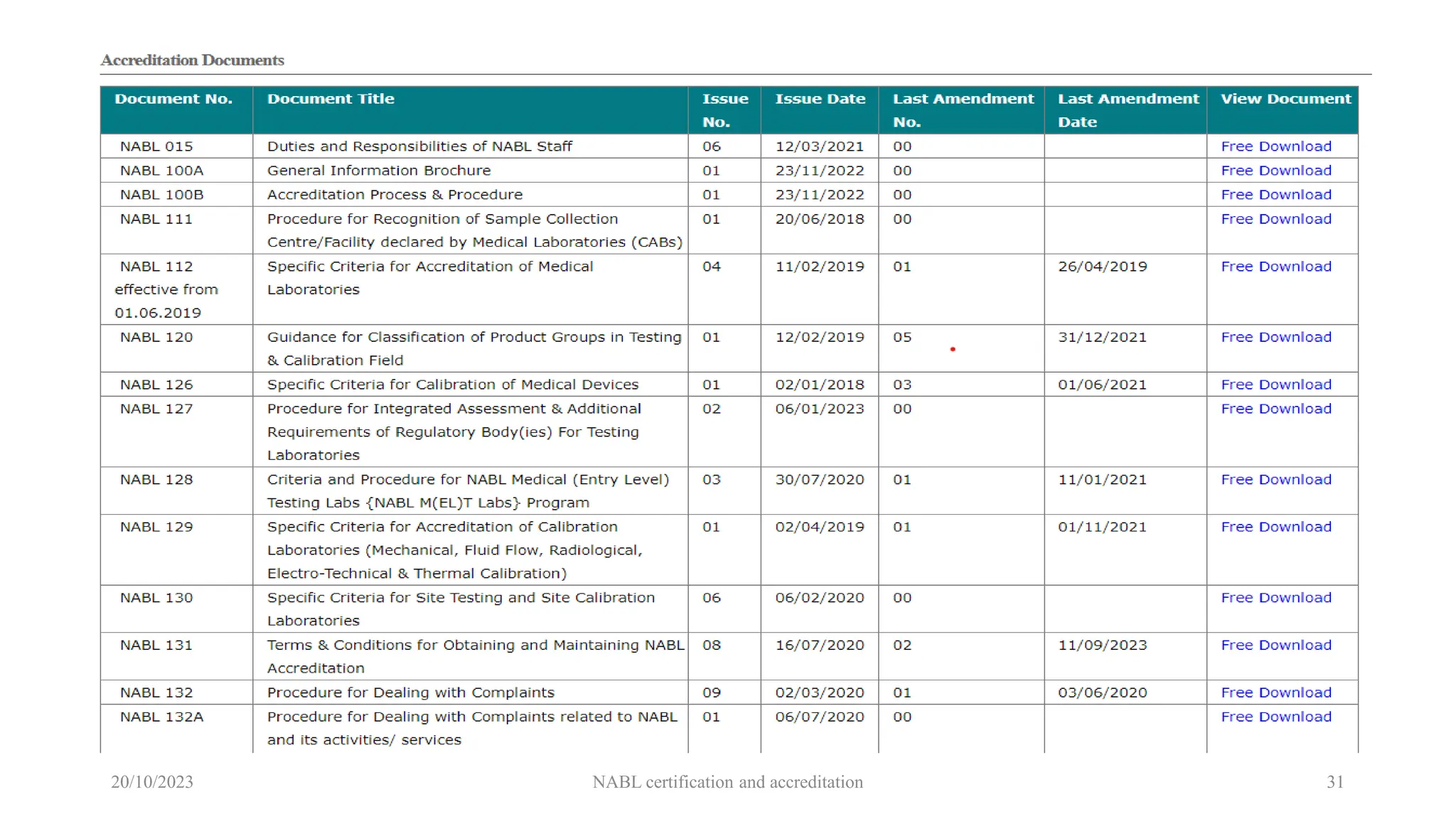
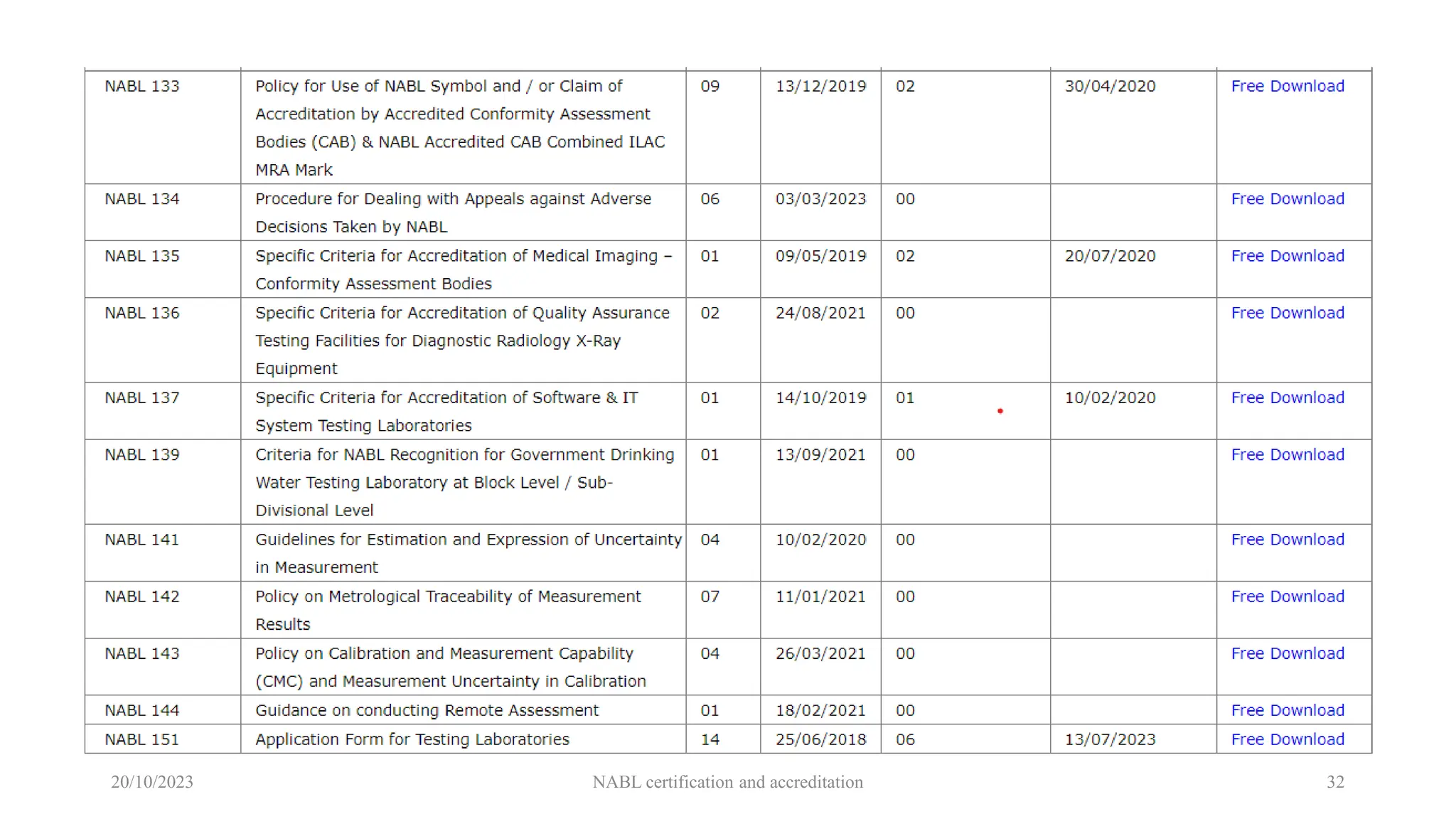
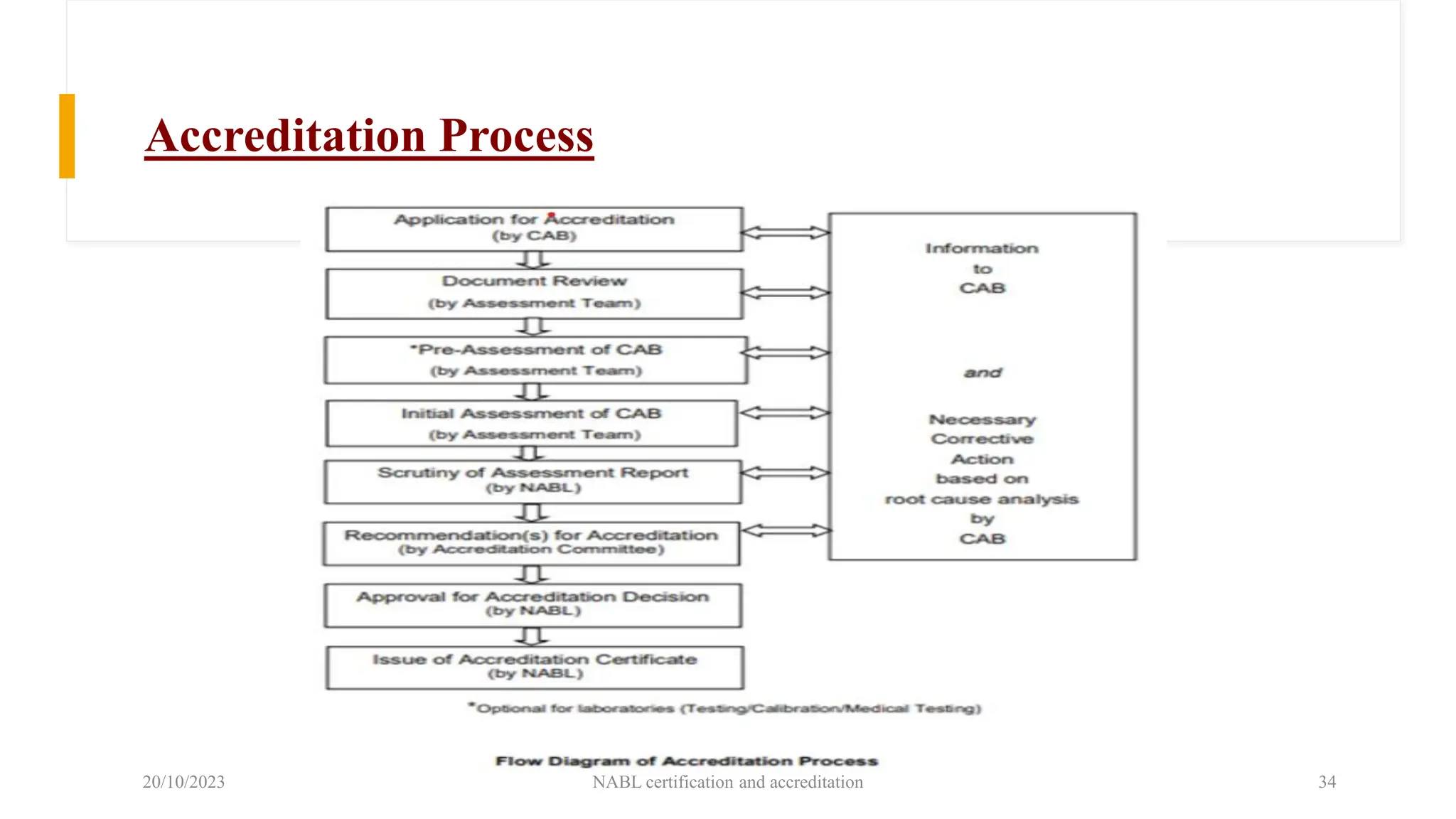
The document provides an overview of the National Accreditation Board for Testing and Calibration Laboratories (NABL), detailing its history, structure, and accreditation services. Established in 1973, NABL offers accreditation for testing and calibration laboratories in line with various ISO standards and is recognized internationally, promoting confidence in testing results across borders. It emphasizes the importance of impartiality in its operations and outlines the benefits of accreditation for laboratories and industry stakeholders.