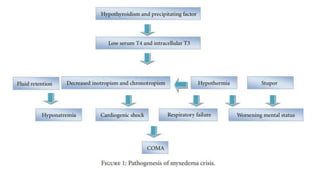
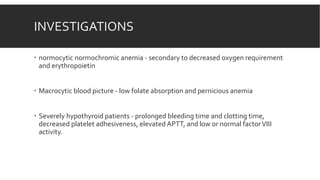
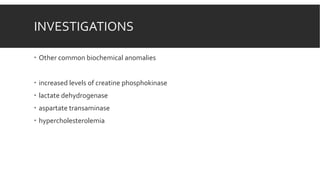
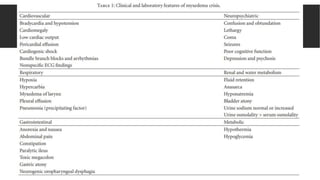
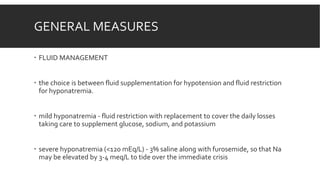
Myxedema coma is a life-threatening complication of severe hypothyroidism. It is characterized by decreased level of consciousness, hypothermia, and other symptoms. Precipitating factors include infection, medications, and cold exposure. Diagnosis involves finding very high TSH and low T4 levels. Treatment requires intensive care, addressing precipitants, fluid management, warming, and thyroid hormone replacement therapy with intravenous T4 or T3. Even with treatment, mortality rates are high at 50-60%.