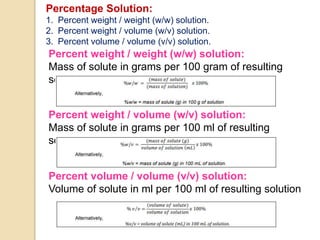
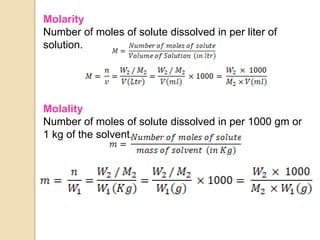
This document discusses different types of solutions and how concentration is expressed. It defines a solution as a homogeneous mixture of two or more substances, with the component present in larger amount called the solvent and the smaller amount called the solute. Concentration expresses the amount of solute dissolved in a given amount of solvent. Some common ways to express concentration include percentage solutions, molarity, molality, normality, and parts per million. Percentage solutions can be expressed as a percentage by mass, percentage by volume, or percentage volume by volume. Molarity is the number of moles of solute per liter of solution, while molality is the number of moles of solute per kilogram of solvent.