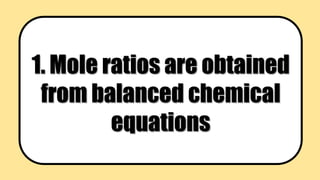
This document provides learning objectives and background information on stoichiometry and mole ratios. The objectives are to calculate amounts of substances used or produced in a chemical reaction using mole ratios. Mole ratios are obtained from balanced chemical equations and can be used to convert between moles of reactants and products. Stoichiometry deals with quantitative relationships between amounts of reactants and products in chemical reactions.