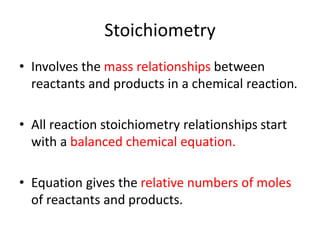
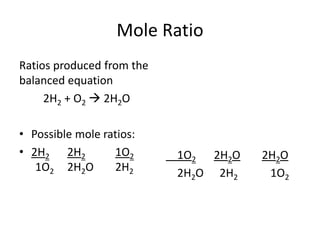
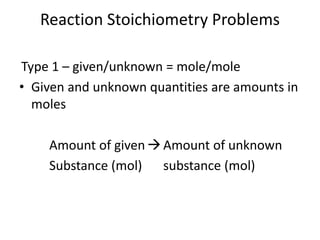
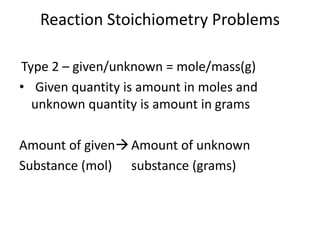
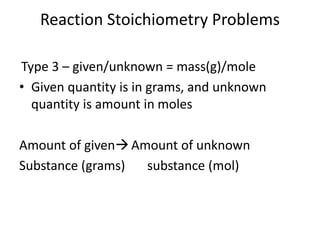
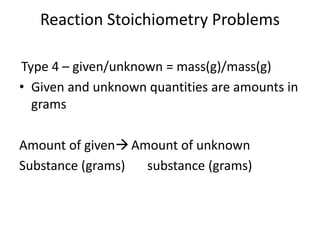
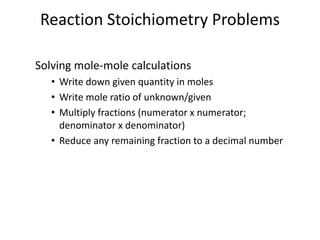
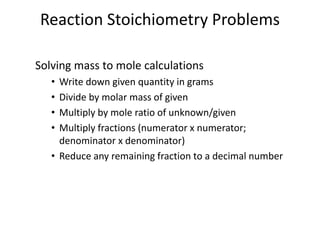
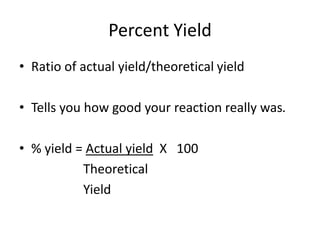
Stoichiometry involves using the mole ratios from a balanced chemical equation to calculate the quantities of reactants and products involved in a chemical reaction. There are four main types of stoichiometry problems involving moles to moles, moles to mass, mass to moles, and mass to mass. Solving these problems uses the mole ratios and molar masses of substances to convert between amounts in moles and grams. The limiting reagent is the reactant that limits the amount of product that can be formed, and the theoretical yield is calculated based on stoichiometry while the actual yield is measured experimentally. The percent yield compares the actual and theoretical yields.