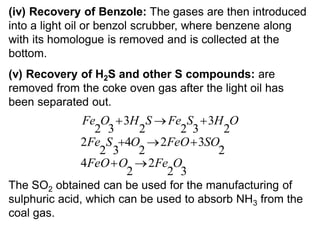
This document summarizes information about solid fuels and the process of carbonization of coal. It discusses how caking coals are heated in the absence of air to produce coke, a solid residue richer in carbon. There are two types of carbonization processes - low temperature carbonization produces semi-coke while high temperature carbonization produces hard metallurgical coke. The document also describes the beehive oven and modern Otto Hoffman oven methods for manufacturing coke and recovering valuable byproducts like tar, ammonia, naphthalene and benzene. Coal washing processes like dense medium separators, jigs and cyclones are also summarized to remove impurities and increase the heating value of coal.