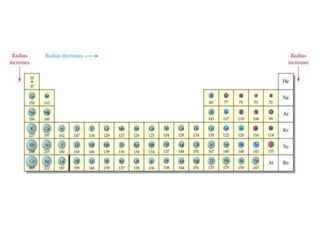
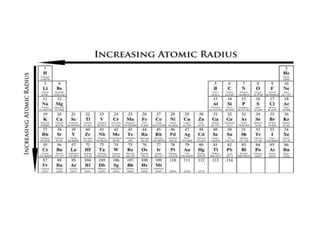
I. Variation of atomic radii along a period and down groups for s-block and p-block elements
(i) Atomic radius decreases from left to right in a period as nuclear charge increases and electrons are pulled closer
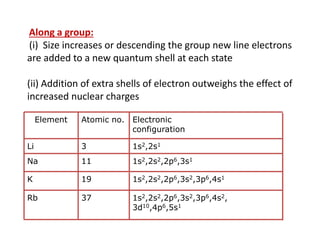
(ii) Atomic radius increases down groups as new electron shells are added and outweigh the effect of increased nuclear charge
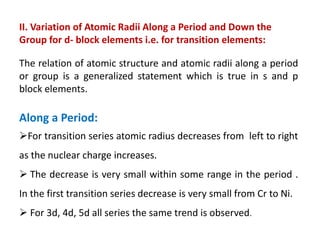
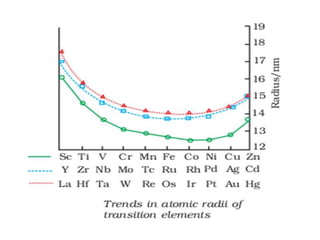
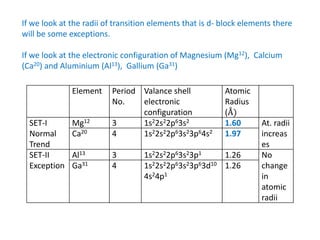
II. Variation trends for d-block transition elements
(i) Atomic radius generally decreases along a period from left to right due to increasing nuclear charge
(ii) Atomic radius increases from 3d to 4d series but is virtually the same from 4d to 5d series due to lanthanide contraction
(iii) Exceptions can occur due to opposing effects