Crystal Structure
- 1. Atomic arrangements in Solids
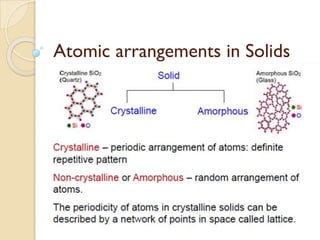
- 2. Crystal: Definition ⚫A crystalline solid is a solid in which the atoms bond with each other in a regular pattern to form a periodic array of atoms. ⚫Crystals are constructed by the infinite repetition of identical structural units in space. ⚫The most important property of a crystal is periodicity, which leads to what is termed long-range order.
- 3. Lattice and Basis ⚫All crystals can be described in terms of a lattice and a basis. ⚫A lattice is an infinite periodic array of geometric points in space, without any atoms. ⚫When we place an identical group of atoms or molecules, called a basis, at each lattice point, we obtain the actual crystal structure.
- 4. Lattice and Basis ⚫ The crystal is thus a lattice plus a basis at each lattice point. ⚫ Crystal = Lattice + Basis ⚫ Crystals with the same lattice but different basis create different crystalline solids but have the same symmetry. ⚫ Here, we have a simple square 2D lattice, and the basis has 2 atoms.
- 5. Unit Cell ⚫ The unit cell is the most convenient building block unit in the crystal structure that carries the properties of the crystal. The repetition of the unit cell in three dimensions generates the whole crystal structure. ⚫ In the previous example, the unit cell is a simple square with two atoms.
- 6. Unit Cell ⚫ Crystals are made up of 3-dimensional arrays of atoms. Since unit cell is the smallest repeating unit having the full symmetry of a crystal structure, crystal structure is described in terms of the geometry of arrangement of particles in the unit cell. ⚫ Unit cells can be visualized in the 2 following ways: ◦ Hard sphere representation: Atoms are denoted by hard, touching spheres showing their realistic arrangement. ◦ Reduced sphere representation: For clarity, it is often more convenient to draw the unit cell with the spheres reduced in size and the distance between spheres highly exaggerated.
- 7. Common Crystal Structures ⚫Simple Cubic (SC) ⚫Body Centered Cubic (BCC) ⚫Face Centered Cubic (FCC)
- 8. Simple Cubic ⚫ The unit cell is cubic with one atom at each of the eight corners. ⚫ Because atoms are shared with neighboring cells, only one- eighth of the corner atoms belong to each unit cell. So the number of atoms per unit cell is: 8 corners x 1/8 = 1 ⚫ Rare due to low packing density (0.52), only Polonium (Po) has this structure. Close packed hard sphere unit cell Reduced sphere unit cell
- 9. BCC Crystals ⚫ The unit cell is cubic with one atom at each of the eight corners and one atom at the center of the cell. ⚫ Because atoms are shared with neighboring cells, only once- eighth of the corner atoms belong to each unit cell. So there are effectively 2 atoms in the space of the unit cell (1 center + 8 corners x 1/8). Close packed hard sphere unit cell Reduced sphere unit cell Note: In a true BCC cell all atoms are identical, the center atom is shaded differently only for ease of viewing.
- 10. BCC Crystals ⚫The body centered atom is in contact with all the eight corner atoms. Each corner atom is shared by eight unit cells and hence, each of these atoms is in touch with eight body centered atoms. ⚫Atoms touch each other along cube diagonals. ⚫Examples: iron, chromium, tungsten, niobium, vanadium, barium. ⚫Volume of the BCC unit cell is 68 percent full of atoms, which is high but lower than the maximum possible packing.
- 11. Generation of BCC structure through unit cell repetition Note: In the simulation some space is allowed between the atoms for better visibility, but originally the atoms in a BCC structures touch each other along cube diagonals.
- 12. FCC Crystals ⚫ The unit cell is cubic with one atom at each of the eight corners and one atom at the center of each of the six faces. ⚫ Because atoms are shared with neighboring cells, only one-eighth of the corner atoms and half of the face centered atom belong to each unit cell. There are effectively 4 atoms in space of the unit cell (6 face x 1/2 + 8 corners x 1/8). Close packed hard sphere unit cell Reduced sphere unit cell
- 13. FCC Crystals ⚫Atoms touch each other along face diagonals. ⚫Examples: copper, nickel, gold, and silver. ⚫Volume of the FCC unit cell is 74 percent full of atoms, which is the maximum possible packing possible with identical spheres. ⚫Also known as Close Packed Cubic (CPC) structure.
- 14. Generation of FCC structure through unit cell repetition Note: In the simulation some space is allowed between the atoms for better visibility, but originally the atoms in a FCC structures touch each other along face diagonals.
- 15. ⮚ The number of nearest neighbours of which an atom has in the unit cell of any crystal structure. Examples ⮚ Simple Cubic (SC) coordination number = 6 ⮚ Body-Centered Cubic(BCC) coordination number = 8 ⮚ Face-Centered Cubic(FCC) coordination number = 12 Coordination Number
- 16. • Coordination # = 6 (# nearest neighbors) SIMPLE CUBIC STRUCTURE (SC)
- 17. Coordination # = 8 BODY CENTERED CUBIC STRUCTURE(BCC)
- 18. • Coordination # = 12 FACE CENTERED CUBIC STRUCTURE (FCC)
- 19. Number of atoms in unit cells
- 20. Atomic Radius Simple Cubic (SC) Lattice
- 21. Atomic Radius of BCC latice
- 23. Atomic Radius of FCC lattice
- 24. Atomic Packing Factor ⚫Atomic packing factor (APF) or packing efficiency indicates how closely atoms are packed in a unit cell and is given by the ratio of volume of atoms in the unit cell and volume of the unit cell.
- 27. =74%
- 28. Unit cell geometry ⚫ To establish a reference frame and to apply three-dimensional geometry, we insert an xyz coordinate system. The x, y, and z axes follow the edges of the parallelepiped and the origin is at the lower-left rear corner of the cell. ⚫ The unit cell extends along the x axis from 0 to a, along y from 0 to b, and along z from 0 to c. ⚫ Conventionally the geometry of the unit cell is represented as a parallelepiped with sides a, b, and c and angles α, β, and γ, as depicted in figure. The sides a, b, and c and angles α, β, and γ are referred to as lattice parameters.
- 29. Miller lndices ⚫ Miller indices of a plane refers to the general convention for describing a particular plane in a crystal based on three-dimensional geometry. ⚫ They are the reciprocals of the (three) axial intercepts for a plane, cleared of fractions & common multiples. ⚫ Algorithm 1) Choose a origin and unit cell of convenience (the plane should not pass through the origin, if it does, shift the origin or draw a plane parallel to it by an appropriate translation). 2) Read off intercepts of plane with axes in terms of a, b, c 3) Take reciprocals of intercepts 4) Clear all fractions without reducing to smallest integer values 5) Enclose in parentheses, no commas i.e., (hkl)
- 30. Miller lndices Example ⚫ Intercepts of the plane are a/2, b, and ∞ along x, y, z axis (the plane is parallel to the z axis) respectively, so intercepts in terms of a, b, c are ½, 1, and ∞. ⚫ Taking reciprocals of intercepts we get 1/(½) , 1/1, 1/ ∞ = 2,1,0. ⚫ This set of numbers does not have fractions, so it is not necessary to clear fractions. ⚫ Hence, the Miller indices (hkl) are (210).
- 31. Miller lndices ⚫ If there is a negative integer due to a negative intercept, a bar is placed across the top of the integer. ⚫ Also, if parallel planes differ only by a shift that involves a multiple number of lattice parameters, then these planes may be assigned the same Miller Indices. indices. For example, the plane (010) is the xz plane that cuts the y axis at —b. If we shift the plane along y by two lattice parameters (2b), it will cut the y axis at b and the Miller indices will become (010). ¯
- 32. Miller lndices ⚫ Planes can have the same Miller indices only if they are separated by an integer multiple of the lattice parameter. For example, the (010) plane is not identical to the (020) plane, even though they are geometrically parallel. The (020) plane cannot be shifted by the lattice parameter b to coincide with plane (010). (010) (020)
- 33. Drawing Planes from Miller Indices ◦ Draw unit cell ◦ Shift origin if necessary. Must if the plane passes through origin, advisable if negative integers present in Miller Indices. (Example: If negative integer is along z axis, move origin along the positive direction of z axis.) ◦ Invert the given indices to get intercepts ◦ Plot the intercepts along the x, y, z axis for non-∞ values.
- 34. Drawing Planes from Miller Indices ◦ For a single ∞ value, plot the other 2 values and connect. Then draw 2 lines along the axis with the ∞ value, starting from the intercept points and ending at the unit cell surface. Connect the points where these lines end at the surface. Example: (110), (101) ¯
- 35. Drawing Planes from Miller Indices ◦ For two ∞ values, plot the single non-∞ value and draw 2 lines along the axes with the ∞ value, starting from the intercept point and ending at the unit cell surface. Draw another 2 lines the same way, but starting from the points where the previous lines ended. Example: (100),(100) ¯
- 36. Example 1.13 ⚫ Consider the FCC unit cell of the copper crystal shown in the figure. a. How many atoms are there per unit cell? b. If R is the radius of the Cu atom, show that the lattice parameter a is given by a = R 2√ 2 c. Calculate the APF. d. Calculate the atomic concentration (number of atoms per unit volume) and the density of the Crystal given that the atomic mass of copper is 63.55 gmol-1 and the radius of the Cu atom is 0.128nm.
- 37. Example 1.13 a) There are four atoms per unit cell. The Cu atom at each corner is shared with eight other adjoining unit cells. Each Cu atom at the face center is shared with the neighboring unit cell. Thus, the number of atoms in the unit cell = 8 corners (1/8 atom) + 6 faces (1/2 atom) = 4 atoms.
- 38. b) Consider one of the cubic faces in above figure. The face is a square of side a and the diagonal is or . The diagonal has one atom at the center of diameter 2R, which touches two atoms at the corners. Example 1.13 The diagonal, going from corner to corner, is therefore R + 2R +R = 4R. ⇒ 4R = ⇒
- 39. Example 1.13 c) From part (b), we know that
- 40. d) In general, atomic concentration is: where x= number of atoms in unit cell. From part (a), we know that x =4 for Cu. Since 1 mole of matter weighs Mat grams and contains NA atoms, each atom weighs Mat/NA grams. Example 1.13
- 41. Example 1.14 a. Find the Miller Indices for the plane shown in the figure which passes through one side of a face and the center of an opposite face in the Cu FCC lattice with a=0.3620 nm. b. Draw (100) and (110) crystallographic planes for the lattice. c. Calculate the planar concentration for each of these planes.
- 42. Example 1.14 Since the plane passes through the origin at the lower-left rear corner, we place the origin to point 0’ at the lower-right rear corner of the unit cell. In terms of lattice parameter a: x, y, and z axes intercepts: ∞, -1, 1/2 reciprocals: 0, -1, 2 Miller indices: (012) ¯
- 43. Example 1.14 ⚫To calculate the planar concentration n(hkl) on a given (hkl) plane, we consider a bound area A of the plane within the unit cell and treat it as the repeat unit in 2D. Only atoms whose centers lie on A are involved in the calculation. ⚫For each atom, we then evaluate what portion of the atomic cross section (a circle in 2D) cut by the plane (hkl) is contained within A.
- 44. Example 1.14 The (100) plane corresponds to a cube face and has an area A = a2. There is one full atom at the center; that is, the (100) plane cuts through one full atom, one full circle in two dimensions, at the face center. However, not all corner atoms are within A. Only a quarter of a circle is within the bound area.
- 45. Example 1.14 ⚫ Number of atoms in A = (4 corners) x 1/4 atom) + 1 atom at face center = 2 ⚫ Planar concentration n(100) of (100) is:
- 46. Example 1.14 ⚫ Consider the (110) plane. The number of atoms in the area A = (a)(a √ 2) defined by two face diagonals and two cube sides is: ⚫ (4 corners) x 1/4 atom) + (2 face diagonals) x (1/2 atom at diagonal center) = 2 ⚫ Planar concentration n(110) of (110) is: