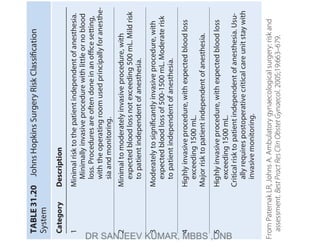
The document describes the Johns Hopkins Surgery Risk Classification System which categorizes surgical procedures into 5 categories based on invasiveness and risk to the patient independent of anesthesia. Category 1 procedures have minimal risk and invasiveness while Category 5 procedures have critical risk and invasiveness, usually requiring post-operative intensive care. It provides descriptions of the expected blood loss, risk level, and typical post-operative care for each category.





![35
•
Neuromuscular
Disorders
Including
Malignant
Hyperthermia
and
Other
Genetic
Disorders
1123
Diagnosis
in
the
Operating
Room
and
Postanesthesia
Care
Unit
As
stated
earlier,
fulminant
MH
is
rare,
and
early
signs
of
clini-
cal
MH
may
be
subtle
(Box
35.1).
These
signs
must
be
distin-
guished
from
other
disorders
with
similar
signs
(Box
35.2).
When
the
diagnosis
is
obvious
(i.e.,
fulminant
MH
or
suc-
cinylcholine-induced
rigidity
with
rapid
metabolic
changes),
marked
hypermetabolism
and
heat
production
occur,
and
there
may
be
little
time
left
for
specific
therapy
to
prevent
death
or
irreversible
morbidity.
If
the
syndrome
begins
with
slowly
increasing
end-tidal
CO
2
(defined
earlier),
specific
ther-
apy
can
await
a
complete
clinical
workup
before
treatment.
In
general,
MH
is
not
expected
to
occur
when
no
triggers
are
administered
(see
“Anesthesia
for
Susceptible
Patients”).
However,
several
confirmed
fulminant
nonanesthetic
cases
of
MH
that
resulted
in
death
have
been
reported
(see
“Non-
anesthetic
Malignant
Hyperthermia”).
148
When
volatile
anesthetics
or
succinylcholine
are
used,
MH
should
be
suspected
whenever
there
is
an
unexpected
increase
in
end-tidal
CO
2
(ETCO
2
),
undue
tachycardia,
tachypnea,
arrhythmias,
mottling
of
the
skin,
cyanosis,
muscle
rigidity,
sweating,
increased
body
temperature,
or
unstable
blood
pressure.
If
any
of
these
occur,
signs
of
increased
metabolism,
acidosis,
or
hyperkalemia
must
be
sought.
The
most
common
cause
for
sudden
ETCO
2
dur-
ing
general
anesthesia
and
sedation
is
hypoventilation.
Increased
minute
ventilation
should
be
able
to
correct
such
a
problem.
The
diagnosis
of
MH
can
be
supported
by
the
analysis
of
arterial
or
venous
blood
gases
which
demonstrates
a
mixed
respiratory
and
metabolic
acidosis;
184
however,
the
respi-
ratory
component
of
acidosis
may
be
predominate
in
the
very
early
stage
of
the
onset
of
fulminant
MH.
O
2
and
CO
2
change
more
markedly
in
the
central
venous
compartment
than
in
arterial
blood;
therefore
end-expired
or
venous
CO
2
levels
more
accurately
reflect
whole-body
stores.
Venous
CO
2
,
unless
the
blood
drains
an
area
of
increased
metabolic
activity,
should
have
PCO
2
levels
of
only
about
5
mm
Hg
greater
than
that
of
expected
or
measured
PaCO
2
.
In
small
children,
particularly
those
without
oral
food
or
fluid
for
a
prolonged
period,
the
base
deficit
may
be
5
mEq/L
because
of
their
smaller
energy
stores.
Any
patient
suspected
of
having
an
MH
episode
should
be
reported
to
the
North
American
MH
Registry
via
the
adverse
metabolic/muscular
reaction
to
anesthesia
(AMRA)
report
available
from
the
website
at
http://anest.ufl.edu/namhr/
namhr-report-forms/.
TREATMENT
Acute
management
for
MH
can
be
summarized
as
follows:
1.
Discontinue
all
triggering
anesthetics,
maintain
intrave-
nous
agents,
such
as
sedatives,
opioids,
and
nondepolar-
izing
muscular
blockers
as
needed,
and
hyperventilate
with
100%
oxygen
with
a
fresh
flow
to
at
least
10
L/min.
With
increased
aerobic
metabolism,
normal
ventilation
must
increase.
However,
CO
2
production
is
also
increased
because
of
neutralization
of
fixed
acid
by
bicarbonate;
hyperventilation
removes
this
additional
CO
2
.
2.
Administer
dantrolene
rapidly
(2.5
mg/kg
intrave-
nously
[IV]
to
a
total
dose
of
10
mg/kg
IV)
every
5
to
10
minutes
until
the
initial
symptoms
subside.
Early
Signs
Elevated
end-tidal
CO
2
Tachypnea
and/or
tachycardia
Masseter
spasm
if
succinylcholine
has
been
used
Generalized
muscle
rigidity
Mixed
metabolic
and
respiratory
acidosis
Profuse
sweating
Mottling
of
skin
Cardiac
arrhythmias
Unstable
blood
pressure
Late
Signs
Hyperkalemia
Rapid
increase
of
core
body
temperature
Elevated
creatine
phosphokinase
levels
Gross
myoglobinemia
and
myoglobinuria
Cardiac
arrest
Disseminated
intravascular
coagulation
BOX
35.1
Clinical
Signs
of
Malignant
Hyperthermia
Anaphylactic
reaction
Alcohol
therapy
for
limb
arteriovenous
malformation
Contrast
dye
injection
Cystinosis
Diabetic
coma
Drug
toxicity
or
abuse
Elevated
end-tidal
CO
2
due
to
laparoscopic
operation
Environmental
heat
gain
more
than
loss
Equipment
malfunction
with
increased
carbon
dioxide
Exercise
hyperthermia
Freeman-Sheldon
syndrome
Generalized
muscle
rigidity
Heat
stroke
Hyperthyroidism
Hyperkalemia
Hypokalemic
periodic
paralysis
Hypoventilation
or
low
fresh
gas
flow
Increased
ETCO
2
from
laparoscopic
surgery
Insufficient
anesthesia
and/or
analgesia
Malignant
neuroleptic
syndrome
Muscular
dystrophies
(Duchenne
and
Becker)
Myoglobinuria
Myotonias
Osteogenesis
imperfecta
Pheochromocytoma
Prader-Willi
syndrome
Recreational
drugs
Rhabdomyolysis
Sepsis
Serotonin
syndrome
Stroke
Thyroid
crisis
Ventilation
problems
Wolf-Hirschhorn
syndrome
BOX
35.2
Conditions
and
Disorders
that
May
Mimic
Malignant
Hyperthermia](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-7-320.jpg)
![SECTION
III
•
Anesthesia
Management
1124
3.
Administer
bicarbonate
(1-4
mEq/kg
IV)
to
correct
the
metabolic
acidosis
with
frequent
monitoring
of
blood
gases
and
pH.
4.
Control
fever
by
administering
iced
fluids,
cooling
the
body
surface,
cooling
body
cavities
with
sterile
iced
fluids,
and
if
necessary,
using
a
heat
exchanger
with
a
pump
oxygenator.
Cooling
should
be
halted
at
38°C
to
prevent
inadvertent
hypothermia.
5.
Monitor
and
treat
arrhythmia.
Advanced
cardiac
life
support
protocol
may
be
applied.
6.
Monitor
and
maintain
urinary
output
to
greater
than
1
to
2
mL/kg/h
and
establish
diuresis
if
urine
output
is
inad-
equate.
Administer
bicarbonate
to
alkalinize
urine
to
pro-
tect
the
kidney
from
myoglobinuria-induced
renal
failure.
7.
Further
therapy
is
guided
by
blood
gases,
electrolytes,
CK,
temperature,
muscle
tone,
and
urinary
output.
Hyperkalemia
should
be
treated
with
bicarbonate,
glu-
cose,
and
insulin,
typically
10
units
of
regular
insulin
and
50
mL
of
50%
dextrose
for
adult
patients.
The
most
effective
way
to
lower
serum
potassium
is
reversal
of
MH
by
effective
doses
(ED)
of
dantrolene.
In
severe
cases,
cal-
cium
chloride
or
calcium
gluconate
may
be
used.
8.
Recent
data
demonstrated
that
magnesium
level
could
be
a
prerequisite
for
dantrolene
efficacy
in
managing
MH
crisis.
9.
Analyze
coagulation
studies
(e.g.,
international
nor-
malized
ratio
[INR],
platelet
count,
prothrombin
time,
fibrinogen,
fibrin
split,
or
degradation
products).
10.
Once
the
initial
reaction
is
controlled,
continued
moni-
toring
in
the
intensive
care
unit
for
24
to
48
hours
is
usually
recommended.
Adequate
personnel
support
is
critical
to
the
successful
management
of
such
a
crisis.
Discontinuation
of
the
trig-
ger
may
be
adequate
therapy
for
acute
MH
if
the
onset
is
slow
or
if
exposure
was
brief.
Changing
the
breathing
cir-
cuit
and
CO
2
absorbent
can
be
time-consuming.
However,
application
of
activated
charcoal
filters
may
rapidly
reduce
the
volatile
anesthetic
concentration
to
an
acceptable
level
in
less
than2
minutes,
if
they
are
readily
available.
185
Dantrolene
used
to
be
packaged
in
20-mg
bottles
with
sodium
hydroxide
for
a
pH
of
9.5
(otherwise
it
will
not
dis-
solve)
and
with
3
g
of
mannitol
(converts
the
hypotonic
solution
to
isotonic).
The
initial
dose
should
be
2.5
mg/
kg
dantrolene
reconstituted
in
sterile
water
and
adminis-
tered
intravenously.
Dantrolene
must
be
reconstituted
in
sterile
water
rather
than
salt
solutions
or
it
will
precipi-
tate.
It
has
been
shown
that
prewarming
of
sterile
water
may
expedite
the
solubilization
of
dantrolene
compared
to
water
in
ambient
temperature.
186
In
2009,
a
newer,
rapid
soluble
lyophilized
powder
form
of
dantrolene
became
available
for
intravenous
use.
It
reconstitutes
in
less
than
a
minute
which
is
much
faster
than
the
older
version.
187
The
higher
dosing
capacity,
250
mg
per
vial,
of
the
newer
version
of
dantrolene
also
reduces
the
stor-
age
space
with
a
similar
recommended
shelf
life
as
the
older
versions.
In
awake,
healthy
volunteers,
the
maximum
twitch
depression
occurs
at
a
dantrolene
dose
of
2.4
mg/kg.
188
Therefore
it
is
not
surprising
that
at
therapeutic
concen-
trations,
dantrolene
may
prolong
the
need
for
intubation
and
assisted
ventilation.
Brandom
and
associates
reviewed
the
complications
associated
with
the
administration
of
dantrolene
from
1987
to
2006
using
the
dataset
in
the
NAMHR
via
the
AMRA
reports
and
found
that
the
most
frequent
complications
of
dantrolene
were
muscle
weak-
ness
(21.7%),
phlebitis
(9%),
gastrointestinal
upset
(4.1%),
respiratory
failure
(3.8%),
hyperkalemia
(3.3%),
and
exces-
sive
secretions
(8.2%).
189
Given
its
high
pH,
it
is
advisable
to
administer
dantrolene
through
a
large
bore
IV
line.
It
has
been
demonstrated
that
dantrolene
interferes
with
EC
coupling
of
murine
intestinal
smooth
muscle
cells,
190
rat
gastric
fundus,
and
colon,
191
which
in
part
explains
its
gastrointestinal
side
effect.
Caution
should
be
used
when
ondansetron
is
to
be
used
in
this
setting.
As
a
serotonin
antagonist,
ondansetron
may
increase
serotonin
at
the
5-HT
2A
receptor
in
the
presynaptic
space.
In
MHS
individu-
als,
agonism
of
5-HT
2A
receptor
may
produce
a
deranged
response,
precipitating
MH.
192
The
clinical
course
will
determine
further
therapy
and
studies.
Dantrolene
should
probably
be
repeated
at
least
every
10
to
15
hours,
since
it
has
a
half-life
of
at
least
10
hours
in
children
and
adults.
188,193
The
total
dose
of
dan-
trolene
that
can
be
used
is
up
to
30
mg/kg
in
some
cases.
Recrudescence
of
MH
can
approach
50%,
usually
within
6.5
hours.
194,195
When
indicated,
calcium
and
cardiac
glycosides
may
be
used
safely.
They
can
be
lifesaving
dur-
ing
persistent
hyperkalemia.
Slow
voltage-gated
calcium
channel
blockers
do
not
increase
porcine
survival.
196,197
Instead,
a
recent
study
by
Migita
demonstrated
that
cal-
cium
channel
blockers,
including
dihydropyridine
(i.e.,
nifedipine),
phenylalkylamine
(i.e.,
verapamil),
and
ben-
zothiazepine
(i.e.,
diltiazem),
led
to
increased
[Ca
2+
]
i
in
human
skeletal
muscle
cells.
Interestingly,
the
potency
of
such
calcium
release
is
correlated
with
the
number
of
binding
sites
on
DHPR
(i.e.,
nifedipine
>
verapamil
>
dil-
tiazem).
198
Clinical
doses
of
dantrolene
were
only
able
to
attenuate
20%
of
the
nifedipine-induced
[Ca
2+
]
i
surge.
198
Current
recommendations
of
MHAUS
discourage
the
use
of
calcium
channel
blockers
in
the
presence
of
dantrolene
because
they
can
worsen
the
hyperkalemia
resulting
in
cardiac
arrest.
Although
administration
of
magnesium
sulfate
could
not
prevent
the
development
of
MH
and
did
not
influence
the
clinical
course
in
succinylcholine-
induced
MH,
199
recent
data
suggested
that
dantrolene
might
require
magnesium
to
arrest
the
course
of
MH
trig-
gered
by
halothane.
200
Permanent
neurologic
sequelae,
such
as
coma
or
paralysis,
may
occur
in
advanced
cases,
probably
because
of
inadequate
cerebral
oxygenation
and
perfusion
for
the
increased
metabolism
and
because
of
the
fever,
acidosis,
hypo-osmolality
with
fluid
shifts,
and
potassium
release.
For
MH
cases
diagnosed
in
the
ambulatory
surgical
cen-
ters,
guidelines
have
been
recently
proposed
for
the
trans-
ferring
of
care
to
receiving
hospital
facilities.
201
Although
it
is
preferable
that
immediate
treatment
and
stabilization
of
the
patient
be
achieved
onsite,
several
factors
need
to
be
considered
before
implementation
of
a
transfer
plan,
which
include
capabilities
of
the
available
professionals
at
the
initial
treatment
and
receiving
facilities,
clinical
best
interests
of
patients,
and
capabilities
of
the
transfer
team.
202
The
validity
of
stocking
dantrolene
in
ambula-
tory
surgery
centers
was
confirmed
with
a
cost-effective-
ness
analysis.
203](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-8-320.jpg)



















![SECTION
III
Anesthesia
Management
1302
Co-oximetry
is
considered
the
gold
standard
for
S
a
O
2
mea-
surements
and
is
relied
on
in
circumstances
when
pulse
oximetry
readings
are
inaccurate
or
unobtainable.
Pulse
Oximetry
Standard
pulse
oximetry
aims
to
provide
a
noninvasive,
in vivo,
and
continuous
assessment
of
functional
S
a
O
2
.
Esti-
mates
of
S
a
O
2
based
on
pulse
oximetry
are
denoted
as
S
p
O
2
.
The
history
of
the
development
of
the
pulse
oximetry
has
been
reviewed
in
detail
elsewhere.
14
Pulse
oximetry
takes
advantage
of
the
pulsatility
of
arte-
rial
blood
flow
to
provide
an
estimate
of
S
a
O
2
by
differentiat-
ing
light
absorption
by
arterial
blood
from
light
absorption
by
other
components.
When
compared
with
in vitro
oximetry
of
an
arterial
blood
sample,
the
challenge
of
obtaining
arterial
O
2
saturation
in vivo
is
to
ensure
that
the
light
is
sampling
arterial
blood
and
to
account
for
its
absorption
by
other
tis-
sues.
As
illustrated
in
Fig.
41.4,
light
absorption
by
tissue
can
be
divided
into
a
time-varying
(pulsatile)
component,
histori-
cally
referred
to
as
“AC”
(from
“alternating
current”),
and
a
steady
(nonpulsatile)
component,
referred
to
as
“DC”
(“direct
current”).
In
conventional
pulse
oximetry,
the
ratio
(R)
of
AC
and
DC
light
absorption
at
two
different
wavelengths
is
calcu-
lated.
The
wavelengths
of
light
are
selected
to
maximize
the
difference
between
the
ratios
of
the
absorbances
of
O
2
Hb
and
deO
2
Hb
(see
Fig.
41.3B).
The
most
commonly
used
wave-
lengths
of
light
are
660
nm
and
940
nm.
At
660
nm,
there
is
greater
light
absorption
by
deO
2
Hb
than
by
O
2
Hb.
At
940
nm,
there
is
greater
light
absorption
by
O
2
Hb
than
by
deO
2
Hb,
(41.5)
where
AC
660
,
AC
940
,
DC
660
,
and
DC
940
denote
the
corre-
sponding
AC
and
DC
components
of
the
640-nm
and
940-
nm
wavelengths.
The
ratio
R
is
then
empirically
related
to
O
2
saturation
based
on
a
calibration
curve
internal
to
each
pulse
oximeter
(Fig.
41.5).
13
Each
manufacturer
develops
its
own
calibration
curve
by
having
volunteers
breathe
hypoxic
gas
mixtures
to
create
a
range
of
S
a
O
2
values
between
70%
and
100%.
The
FDA
0
5
10
15
20
25
650
600
550
500
700
nm
Methemoglobin
Oxyhemoglobin
Reduced
hemoglobin
Carboxyhemoglobin
Sulfhemoglobin
0.01
10
1
0.1
920
960
600
640
680
720
Wavelength
(nm)
760
800
840
880
Extinction
coefficient
Red
Infrared
Methemoglobin
Oxyhemoglobin
Reduced
hemoglobin
Carboxyhemoglobin
A
B
Wavelength
(nm)
Millimolar
absorptivity
Fig.
41.3
(A)
Absorption
spectra
of
five
species
of
hemoglobin
for
wavelengths
of
light
across
the
visible
spectrum.
(B)
Extinction
coefficients
of
the
most
frequently
measured
hemoglobin
species
extending
to
infrared
wavelengths
used
for
pulse
oximetry.
The
vertical
lines
indicate
specific
wave-
lengths
for
red
and
infrared
light
applied
in
pulse
oximeters.
The
differences
in
the
extinction
coefficients
of
oxyhemoglobin
and
reduced
hemoglobin
(deoxygenated
hemoglobin)
are
pronounced
at
these
wavelengths.
Note
that
the
extinction
coefficients
of
carboxyhemoglobin
and
methemoglobin
are
similar
to
those
of
oxyhemoglobin
and
reduced
hemoglobin,
respectively,
at
660
nm.
([A]
Redrawn
from
Zwart
A,
van
Kampen
EJ,
Zijlstram
WG.
Results
of
routine
determination
of
clinically
significant
hemoglobin
derivatives
by
multicompartment
analysis.
Clin
Chem.
1986;32:972–978.
[B]
Modified
from
Trem-
per
KK,
Barker
SJ.
Pulse
oximetry.
Anesthesiology.
1989;70:98–108.)
AC
Absorption
from
pulsatile
arterial
blood
Absorption
from
nonpulsatile
arterial
blood
Absorption
from
venous
and
capillary
blood
Absorption
from
tissue
DC
Time
Light
absorption
Fig.
41.4
Schematic
of
the
Pulse
Principle.
Absorption
of
light
passing
through
tissue
is
characterized
by
a
pulsatile
component
(AC)
and
a
nonpul-
satile
component
(DC).
The
pulsatile
component
of
absorption
is
due
to
arterial
blood.
The
nonpulsatile
component
is
due
to
venous
blood
and
the
remainder
of
the
tissues.
(Redrawn
from
Severinghaus
JW.
Nomenclature
of
oxygen
saturation.
Adv
Exp
Med
Biol.
1994;345:921–923)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-28-320.jpg)


![Respiratory
Monitoring
1313
˙
˙
mismatch
is
the
most
common
cause
of
hypoxemia
in
the
clinical
setting.
Ventilation
and
perfusion
are
non-
uniformly
distributed
throughout
the
normal
lung,
with
worsening
mismatch
in
the
setting
of
lung
disease,
general
anesthesia,
and
mechanical
ventilation.
Areas
with
low
or
zero
˙
˙
yield
low
end-capillary
PO
2
,
whereas
areas
with
normal
or
high
˙
˙
produce
higher
end-capillary
PO
2
.
However,
because
of
the
plateau
of
the
O
2
Hb
dissociation
curve
(see
Fig.
41.2),
the
normal
and
high
˙
˙
regions
are
limited
in
the
extent
to
which
they
increase
the
O
2
content
and
compensate
for
the
low
˙
˙
regions
(Fig.
41.15).
Con-
sequently,
˙
˙
mismatch
results
in
hypoxemia.
Right-to
left
shunt
is
the
amount
of
blood
that
flows
from
the
pulmonary
artery
to
the
systemic
arterial
circulation
without
undergoing
pulmonary
gas
exchange.
It
represents
an
extreme
case
of
˙
˙
mismatch
in
which
the
ratio
equals
zero
and
the
end-capillary
gas
partial
pressures
are
equal
to
the
values
found
in
mixed
venous
blood.
In
healthy
awake
spontaneously
breathing
subjects,
intrapulmonary
shunt
is
negligible,
179
and
a
small
(<1%
of
cardiac
output)
extrapul-
monary
shunt
results
from
drainage
of
the
bronchial
and
Thebesian
veins
into
the
arterial
side
of
the
circulation.
180
During
general
anesthesia,
a
right-to-left
shunt
can
develop
as
a
result
of
atelectasis.
181,182
Right-to-left
shunt
can
also
be
seen
in
pathologic
conditions
such
as
pneumonia
and
acute
lung
injury.
The
effect
of
the
shunt
on
P
a
O
2
is
a
func-
tion
of
the
magnitude
of
the
shunt,
F
I
O
2
,
and
the
cardiac
output
(Fig.
41.16).
Importantly,
increases
in
F
I
O
2
have
a
small
effect
on
P
a
O
2
in
the
presence
of
large
true
right-to-left
shunt
(see
Fig.
41.16).
The
traditional
method
to
estimate
flow
through
shunt-
ing
regions
(
˙
)
as
a
fraction
of
the
total
cardiac
output
(
˙
)
is
based
on
the
modeling
of
the
lung
as
a
three-compartment
system
(Fig.
41.17).
183
The
three
compartments
represent
(1)
lung
regions
receiving
both
ventilation
and
perfusion,
TABLE
41.2
Causes
of
Changes
in
Partial
Pressure
of
End-Tidal
Carbon
Dioxide
↑P
ET
CO
2
↓P
ET
CO
2
↑CO
2
Production
and
Delivery
to
the
Lungs
Increased
metabolic
rate
Fever
Sepsis
Seizures
Malignant
hyperthermia
Thyrotoxicosis
Increased
cardiac
output
(e.g.,
during
CPR)
Bicarbonate
administration
↓CO
2
Production
and
Delivery
to
the
Lungs
Hypothermia
Pulmonary
hypoperfusion
Cardiac
arrest
Pulmonary
embolism
Hemorrhage
Hypotension
↓Alveolar
Ventilation
Hypoventilation
Respiratory
center
depression
Partial
muscular
paralysis
Neuromuscular
disease
High
spinal
anesthesia
COPD
↑Alveolar
Ventilation
Hyperventilation
Equipment
Malfunction
Rebreathing
Exhausted
CO
2
absorber
Leak
in
ventilator
circuit
Faulty
inspiratory/expiratory
valve
Equipment
Malfunction
Ventilator
disconnect
Esophageal
intubation
Complete
airway
obstruction
Poor
sampling
Leak
around
endotracheal
tube
cuff
CO
2
,
Carbon
dioxide;
COPD,
chronic
obstructive
pulmonary
disease;
CPR,
cardiopulmonary
resuscitation;
P
ET
CO
2
,
partial
pressure
of
end-tidal
carbon
dioxide.
Modified
from
Hess
D.
Capnometry
and
capnography:
technical
aspects,
physiologic
aspects,
and
clinical
applications.
Respir
Care.
1990;35:557–576.
Increased
ventilation-perfusion
heterogeneity,
particularly
with
high
V/Q
regions
Pulmonary
hypoperfusion
Pulmonary
embolism
Cardiac
arrest
Positive
pressure
ventilation
(especially
with
PEEP)
High-rate
low-tidal-volume
ventilation
BOX
41.2
Causes
of
Increased
Arterial-
to-End-Tidal
Carbon
Dioxide
Pressure
Difference
P
(a-ET)CO2
PEEP,
Positive
end-expiratory
pressure.
Modified
from
Hess
D.
Capnom-
etry
and
capnography:
technical
aspects,
physiologic
aspects,
and
clinical
applications.
Respir
Care.
1990;35:557–576.
Expired
volume
Z
Y
X
q
p
F
CO
2
F
ET
CO
2
F
CO
2
of
a
gas
in
equilibrium
with
arterial
blood
V
D
aw
V
T
alv
V
T
Fig.
41.13
The
volume
capnogram
is
a
plot
of
the
fraction
of
CO
2
(FCO
2
)
in
exhaled
gas
versus
exhaled
volume.
It
is
divided
into
three
phases,
which
reflect
the
same
sources
of
expired
gas
as
present
in
the
time
capnograph:
anatomic
dead
space
(phase
I,
red),
transitional
(phase
II,
blue),
and
alveolar
gas
(phase
III,
green).
The
volume
capno-
gram
allows
for
the
partition
of
total
tidal
volume
(V
T
)
into
airway
dead
space
volume
(V
D
aw)
and
an
effective
alveolar
tidal
volume
(V
T
alv)
by
a
vertical
line
through
Phase
II,
positioned
such
that
the
approximately
triangular
areas
p
and
q
are
equal.
It
also
provides
the
slope
of
phase
III
as
a
quantitative
measure
of
the
heterogeneity
of
alveolar
ventila-
tion.
The
total
area
below
the
horizontal
line
(denoting
the
FCO
2
of
a
gas
in
equilibrium
with
arterial
blood)
can
be
divided
into
three
dis-
tinct
areas:
X,
Y,
and
Z.
Area
X
corresponds
to
the
total
volume
of
CO
2
exhaled
over
a
tidal
breath.
This
value
can
be
used
to
compute
the
CO
2
production
(
V
CO
2
),
and
the
mixed
expired
CO
2
fraction
or
partial
pres-
sure
to
be
used
in
the
Bohr
equation
(Eq.
[41.15])
based
on
the
divi-
sion
of
the
exhaled
CO
2
volume
by
the
exhaled
tidal
volume.
Area
Y
represents
wasted
ventilation
due
to
alveolar
dead
space,
while
area
Z
corresponds
to
wasted
ventilation
due
to
anatomic
deadspace
(V
D
aw).
Thus
areas
Y
+
Z
represent
the
total
physiologic
dead
space.
The
vol-
ume
capnogram
can
also
be
plotted
as
a
PCO
2
versus
exhaled
volume
curve.
F
ET
CO
2
,
Fraction
of
end-tidal
carbon
dioxide.
(Modified
from
Fletcher
R,
Jonson
B,
Cumming
G,
et
al.
The
concept
of
deadspace
with
special
reference
to
the
single
breath
test
for
carbon
dioxide.
Br
J
Anaesth.
1981;53:77–88.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-31-320.jpg)


![Neuromuscular
Monitoring
1371
Reversal
with
sugammadex
(SUG)
Reversal
with
neostigmine
(NEO)
PTC
0
PTC
1–15
TOF
0.9
No
response
to
TOF
TOF
ratio
1.0
Reversal
when
quantitative
(objective)
neuromuscular
monitoring
is
available
and
reliable
SUG
16
mg/kg
SUG
4
mg/kg
SUG
2
mg/kg
SUG
2
mg/kg
No
reversal
NEO
0.05
mg/kg
NEO
0.02
mg/kg
Delay
reversal
to
the
TOF
count
of
2
TOF
count
1–4
TOF
0.4
or
TOF
count
2–3
TOF
0.9
TOF
0.9
TOF
0.4–0.9
TOF
0–1
Reversal
with
sugammadex
(SUG)
Reversal
with
neostigmine
(NEO)
PTC
0
PTC
1–15
No
response
to
TOF
No
response
to
TOF
TOF
count
1–4
with
or
without
fade
Reversal
when
peripheral
(subjective)
nerve
stimulator
is
available
only
or
quantitative
neuromuscular
monitoring
is
unreliable
SUG
16
mg/kg
SUG
4
mg/kg
With
fade
Without
fade
NEO
0.04
mg/kg
NEO
0.02
mg/kg
SUG
2
mg/kg
NEO
0.05
mg/kg
Delay
reversal
to
the
TOF
count
of
2
TOF
count
4
TOF
count
2–3
Fig.
43.20
Suggestion
to
diminish
the
incidence
of
residual
curarization
by
neostigmine
(NEO)
or
sugammadex
(SUG)
according
to
the
level
of
block,
determined
with
a
nerve
stimulator
(quantitative
or
peripheral).
Note
that
only
a
quantitative
measured
TOF
ratio
of
0.90
to
1.00
ensures
low
risk
of
clinically
significant
residual
block.
PTC,
Posttetanic
count;
TOF,
train-of-four.
(Modified
from
Kopman
AF,
Eikermann
M.
Antagonism
of
non-depolarising
neuromuscular
block:
current
practice.
Anaesthesia.
2009;64[Suppl
1]:22–30.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-34-320.jpg)

















![Respiratory
Disorders
Acute
Respiratory
Acidosis
Expected
[
HCO
−
3
]
=
24
+
[(
measured
PaCO
2
−
40
)
/10
]
Chronic
Respiratory
Acidosis
Expected
[
HCO
−
3
]
=
24
+
4
[(
measured
PaCO
2
−
40
)
/10
]
Acute
Respiratory
Alkalosis
Expected
[
HCO
−
3
]
=
24
−
2
[(
40
−
measured
PaCO
2
)
/10
]
Chronic
Respiratory
Alkalosis
Expected
[
HCO
−
3
]
=
24
−
5
[(
40
−
measured
PaCO
2
)
/10
]
(
range:
±
Metabolic
Disorders
Metabolic
Acidosis
Expected
PaCO
2
=
1
.
5
×
[
HCO
−
3
]
+
8
(
range
:
±
2
)
Metabolic
Alkalosis
Expected
PaCO
2
=
0
.
7
[
HCO
−
3
]
+
20
(
range
:
±
5
)
BOX
48.1
The
Descriptive
(CO
2
−HCO
3
−
)
Approach
to
Acid-Base](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-52-320.jpg)
![THE
SEMI-QUANTITATIVE
(BASE
DEFICIT/
EXCESS
[COPENHAGEN])
APPROACH
In
metabolic
acidosis,
the
addition
of
UMA
to
the
ECF
results
in
a
net
gain
of
one
hydrogen
ion
for
each
anion.
This
is
buffered,
principally
by
bicarbonate,
such
that
each
anion
gained
results
in
an
equivalent
fall
in
the
bicarbonate
con-
centration.
Thus,
the
change
in
the
bicarbonate
concentra-
tion
from
baseline
should
reflect
the
total
quantity
of
net
Delta
ratio
=
ΔAnion
gap/Δ
[
HCO
−
3
]
or
↑
anion
gap/
↓
[
HCO
−
3
]
=
Measured
anion
gap–
Normal
anion
gap
Normal
[
HCO
−
3
]
–
Measured
[
HCO
−
3
]
=
(
AG
–
12
)
(
24
−
[
HCO
−
3
])
BOX
48.2
The
Delta
Anion
Gap
(Delta
Ratio)
Acidosis
<7.35
Anion
gap
Delta
ratio
Correct
for
albumin
Acute
Chronic
Alkalosis
>7.45
pH
Delta
ratio
Clinical
assessment
<0.4
<1
1
to
2
>2
Hyperchloremic
normal
AG
acidosis
High
AG
and
normal
AG
acidosis
Pure
anion
gap
acidosis
Lactic
acidosis:
average
value
1.6
DKA
more
likely
to
have
a
ratio
closer
to
1
because
of
urine
ketone
loss
High
AG
acidosis
and
concurrent
metabolic
alkalosis
or
preexisting
compensated
respiratory
acidosis
Metabolic
acidosis
Pa
CO
2
Pa
CO
2
Pa
CO
2
Pa
CO
2
=
Pa
CO
2
Respiratory
alkalosis
Pa
CO
2
Respiratory
acidosis
Pa
CO
2
Metabolic
alkalosis
Pa
CO
2
Fig.
48.4
The
Descriptive
(“Boston”)
Approach
to
Acid-Base
Balance.
DKA,
Diabetic
ketoacidosis;
AG,
Anion
gap.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-53-320.jpg)

![Perioperative
Acid-Base
Balance
1535
There
has
been
considerable
discussion
over
the
past
60
years
about
the
merits
and
demerits
of
the
BE,
as
compared
to
the
CO
2
-HCO
3
−
system.
In
reality,
there
is
little
difference
between
the
two;
both
equations
and
nomograms
were
derived
from
patient
data
and
abstracted
backward.
Cal-
culations
use
bicarbonate
as
measured
on
a
blood
gas
ana-
lyzer.
Consequently,
for
most
patients,
either
approach
is
relatively
accurate
but
may
be
misleading
because
they
do
not
allow
the
clinician
to
distinguish
between,
for
exam-
ple,
acidosis
due
to
lactate
or
chloride,
or
alkalosis
due
to
dehydration
or
hypoalbuminemia.
These
measures
may
miss
the
presence
of
an
acid-base
disturbance
entirely;
for
example,
a
hypoalbuminemic
(metabolic
alkalosis)
criti-
cally
ill
patient
with
a
lactic
acidosis
(metabolic
acidosis)
may
have
a
normal
range
pH,
bicarbonate,
and
BE.
This
lack
of
precision
may
lead
to
inappropriate
or
inadequate
therapy.
Changes
in
the
BE
occur
secondary
to
alterations
in
the
relative
concentrations
of
sodium,
chloride,
free
water,
albumin,
phosphate,
and
UMA.
By
calculating
the
contribution
of
the
individual
components
of
the
BE
it
is
possible
to
identify:
(1)
contraction
alkalosis,
(2)
hypo-
albuminemic
alkalosis,
(3)
hyperchloremic
acidosis,
(4)
dilution
acidosis
(if
indeed
it
exists),
and
(5)
acidosis
sec-
ondary
to
UMA.
This
approach,
which
can
be
labelled
the
base-deficit
gap,
has
been
proposed
by
Gilfix
and
Magder
(see
Box
48.3)
58
and
simplified
subsequently
by
Balasu-
bramanyan
and
associates
59
and
Story
and
associates.
60
The
BDG
should
mirror
the
SIG
(below)
the
corrected
AG.
The
simplified
calculation
as
proposed
by
Story
et al.
is
very
easy
to
calculate
at
the
bedside
and
in
the
majority
of
situations
(see
Box
48.3)
replicates
the
more
complex
calculations
originally
proposed
by
Gilfix
and
Magder.
58
STEWART
APPROACH
A
more
accurate
reflection
of
true
acid-base
status
can
be
derived
using
the
Stewart
or
physical
chemi-
cal
approach,
subsequently
updated
by
Fencl.
5,15
This
approach
is
based
on
the
concept
of
electrical
neutrality,
a
small
advance
from
the
AG.
There
exists,
in
plasma,
a
(
)
of
40
to
44
mEq/L,
balanced
by
the
negative
charge
on
bicar-
bonate
and
A
TOT
(the
BB—SIDe).
There
is
a
small
difference
between
SIDa
(apparent
SID)
and
BB
(SIDe—effective
SID)
that
represents
a
SIG
and
quantifies
the
amount
of
UMA
present
(Fig.
48.7).
(
)
[
]
[
]
[
]
(
)
[
]
[
]
[
]
(
)
Weak
acids’
degree
of
ionization
is
pH
dependent,
so
one
must
calculate
for
this:
[
]
[
]
(
.
)
Pi
(mmol/L)
=
[PO
4
]
×
(0.309
×
pH
–
0.469)
(
)
Unfortunately,
the
SIG
may
not
represent
unmeasured
strong
anions
but
only
all
anions
that
are
unmeasured.
For
example,
if
a
patient
has
been
resuscitated
with
gelatin,
his/
her
SIG
will
increase.
Further,
SID
changes
quantitatively
in
absolute
and
relative
terms
when
there
are
changes
in
plasma
water
concentration.
Fencl
has
addressed
this
by
correcting
the
chloride
concentration
for
free
water
(Cl
−
corr)
using
the
following
equation
5
:
[Cl
−
]
corr
=
[Cl
−
]
observed
×
([Na
+
]normal
/
[Na
+
]observed
).
This
corrected
chloride
concentration
may
then
be
inserted
into
the
SIDa
equation
above.
Similarly,
the
derived
value
for
UMA
can
also
be
corrected
for
free
water
by
sub-
stituting
UMA
for
Cl
−
in
the
above
equation.
25
In
a
series
of
nine
normal
subjects,
Fencl
estimated
the
“normal”
SIG
as
8
±
2
mEq/L.
25
Calculation
of
SIG
is
cumbersome.
The
data
required
are
more
extensive
and
thus
more
expensive
than
other
approaches
and
there
is
much
confusion
about
the
normal
range
of
SIG.
It
is
unclear,
in
standard
clinical
practice,
that
SIG
has
any
advantage
over
AGc
(which
is
SIG
without
cal-
cium,
magnesium,
and
phosphate—which
usually
cancel
out
each
other’s
charges).
In
all
likelihood
no
single
number
will
ever
allow
us
to
make
sense
of
complex
acid-base
disturbances.
Fencl
25
has
suggested
that,
rather
than
focusing
on
AG
or
BDE,
physicians
should
address
each
blood
gas
in
terms
of
all
alkalinizing
and
acidifying
effects:
respiratory
acidosis/
alkalosis,
the
presence
or
absence
of
abnormal
SID
(due
to
BE
NaW
(
water
and
sodium
effect
)
=
0.3
([
Na
+
meas
]
-140
)
mEq/L
BE
Cl
(
chloride
effect
)
=
102
−
[
Cl
−
effective
]
(
mEq/L
)
BE
Pi
(
phosphate
effect
)
=
(
0
.
309
×
(
pH
–
0
.
47
))
×
Pi
mEq/L
BE
prot
(
protein
effect
)
=
(
42
−
[
Albumin
g/L
])
*
(
0.148
×
pH
−
0.818
)
BE
calc
=
NE
NaW
+
BE
Cl
+
BE
PO4
+
BE
prot
BE
Gap
=
BE
calc
–
BE
actual
–
[
lactate
mEq/L
]
A
Simplified
Calculation
of
the
Base
Excess
Gap
60
BE
NaCl
=
([
Na
+
]
–
[
Cl
–
])
–
38
BE
Alb
=
0.25
(
42
–
albumin
g/L
)
BE
NaCl
–
BE
Alb
=
BDE
calc
BE
actual
–
BE
calc
–
[
lactate
]
=
BEG=
the
effect
of
unmeasured
anions
or
cations
BOX
48.3
Calculation
of
the
Base
Excess
Gap
12,58,59
*This
approach
involves
calculating
the
base
deficit
excess
for
sodium,
chloride,
and
free
water
(BE
NaCl
),
and
that
for
albumin
(BE
Alb
).
The
re-
sult
is
the
calculated
BE
(BE
calc
).
This
is
subtracted
from
the
measured
BE
to
find
the
BE
gap.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-55-320.jpg)





![□
Conventional
perioperative
analgesia
regimens
do
not
meet
the
needs
of
the
chronic
pain
patient.
□
Unrelieved
postoperative
pain
due
to
undermedication
may
provoke
withdrawal.
□
Patients
tend
to
underreport
their
medication.
□
With
uncontrolled
anxiety
or
fear
of
pain,
patients
tend
to
over-
estimate
the
effect
of
painful
stimuli.
□
Epidural
and
intravenous
(IV)
opioid
(including
patient-con-
trolled
analgesia
[PCA])
requirements
can
be
2-4
times
higher
in
opioid-consuming
than
in
opioid-naïve
patients.
□
Expect
prolonged
recovery
and
need
for
postoperative
analge-
sia.
□
Anxiety
and
insufficient
coping
result
in
poor
compliance
with
analgesic
strategies.
□
Individual
variations
in
response
to
opioids
may
necessitate
selection
of
the
optimal
drug
and
dosing
by
sequential
trials.
□
Individual
titration
of
doses
to
find
the
optimal
balance
be-
tween
analgesia
and
adverse
effects
is
required.
□
Adjuvant
medication
may
interfere
with
anesthesia
and
postop-
erative
analgesia.
BOX
51.1
Risk
Factors
in
the
Perioperative
Management
of
the
Chronic
Pain
Patient
Adapted
from
Kopf
A,
Banzhaf
A,
Stein
C.
Perioperative
manage-
ment
of
the
chronic
pain
patient.
Best
Pract
Res
Clin
Anaesthesiol.
2005;19:59–76.
202](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-61-320.jpg)



![Palliative
Medicine
1633
the
physician
and
patient
or
family
agree
to
reevaluate
the
benefit
after
a
specified
period,
may
be
helpful.
94
Time-
limited
trials
give
families
a
sense
of
when
the
health-
care
team
expects
to
know
whether
an
intervention
is
helping
and
creates
the
expectation
that
the
issue
will
be
readdressed.
RESUSCITATION
STATUS
Outcomes
of
Cardiopulmonary
Resuscitation
CPR
was
introduced
around
1960,
initially
as
a
treatment
for
intraoperative
events
95
and
then
expanded
outside
the
surgical
unit.
Outcomes
of
CPR
have
improved,
with
over
PATIENT-TESTED
LANGUAGE
"I'd
like
to
talk
about
what
is
ahead
with
your
illness
and
do
some
thinking
in
advance
about
what
is
important
to
you
so
that
I
can
make
sure
we
provide
you
with
the
care
you
want
––
is
this
okay?"
"What
is
your
understanding
now
of
where
you
are
with
your
illness?"
"How
much
information
about
what
is
likely
to
be
ahead
with
your
illness
would
you
like
from
me?"
"I
want
to
share
with
you
my
understanding
of
where
things
are
with
your
illness
...
"
Uncertain:
"It
can
be
difficult
to
predict
what
will
happen
with
your
illness.
I
hope
you
will
continue
to
live
well
for
a
long
time
but
I'm
worried
that
you
could
get
sick
quickly,
and
I
think
it
is
important
to
prepare
for
that
possibility."
OR
Time:
"I
wish
we
were
not
in
this
situation,
but
I
am
worried
that
time
may
be
as
short
as
(express
as
a
range,
e.g.
days
to
weeks,
weeks
to
months,
months
to
a
year)."
OR
Function:
"I
hope
that
this
is
not
the
case,
but
I'm
worried
that
this
may
be
as
strong
as
you
will
feel,
and
things
are
likely
to
get
more
difficult."
"What
are
your
most
important
goals
if
your
health
situation
worsens?"
"What
are
your
biggest
fears
and
worries
about
the
future
with
your
health?"
"What
gives
you
strength
as
you
think
about
the
future
with
your
illness?"
"What
abilities
are
so
critical
to
your
life
that
you
can't
imagine
living
without
them?"
"If
you
become
sicker,
how
much
are
you
willing
to
go
through
for
the
possibility
of
gaining
more
time?"
"How
much
does
your
family
know
about
your
priorities
and
wishes?"
"I've
heard
you
say
that
is
really
important
to
you.
Keeping
that
in
mind,
and
what
we
know
about
your
illness,
I
recommend
that
we
.
This
will
help
us
make
sure
that
your
treatment
plans
reflect
what's
important
to
you."
"How
does
this
plan
seem
to
you?"
"I
will
do
everything
I
can
to
help
you
through
this."
SET
UP ASSESS SHARE EXPLORE CLOSE
Fig.
52.7
Serious
illness
conversation
guide.
(2015
to
2017
Ariadne
Labs:
A
Joint
Center
for
Health
Systems
Innovation
[www.ariadnelabs.org]
between
Brigham
and
Women’s
Hospital
and
the
Harvard
T.H.
Chan
School
of
Public
Health,
in
collaboration
with
Dana-Farber
Cancer
Institute.
Licensed
under
the
Creative
Commons
Attribution-NonCommercial-ShareAlike
4.0
International
License,
http://creativecommons.org/licenses/by-
nc-sa/4.0/.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-66-320.jpg)


































![Anesthesia
for
Cardiac
Surgical
Procedures
1739
wall
motion
abnormalities.
Reopening
the
chest
may
be
necessary
while
treatment
is
instituted.
Occasionally,
the
patient’s
sternum
cannot
be
closed
because
of
hemo-
dynamic
instability;
in
such
cases,
only
skin
closure
is
attempted,
and
plans
are
made
to
return
to
the
operat-
ing
room
for
sternal
wiring
after
a
period
of
myocardial
recovery
in
the
ICU.
TRANSPORT
TO
THE
INTENSIVE
CARE
UNIT
Transportation
of
postcardiac
surgical
patients
to
the
ICU
is
frequently
dangerous
and
underestimated.
Prepara-
tion
for
transportation
of
a
postcardiac
surgical
patient
should
start
with
evaluation
of
the
stability
of
the
patient
in
the
operating
room.
An
ICU
bed
with
a
portable
-
Checklist
1.
Rectal/bladder
T˚
>
35.5˚
C
2.
Hct
25%,
K
+
3.8–5
mEq,
pH
>
7.3,
glycemia
6–9
mm/L
3.
Surgeon
→
aortic
unclamping
±
De-airing
cardiac
cavities
±
Defibrillation
4.
Lung
reventilation
5.
Spontaneous
or
paced
HR
(>
70
beats/min)
6.
TEE
examination
under
partial
CPB
→
Check
for
structural
defects
→
Check
for
functional
defects
Step
1
Hemodynamic
targets
Step
3
Mechanical
circulatory
support
→
Successful
CPB
weaning
Protamine
IV
and
ACT
control
Inability
to
wean
from
CPB
despite
preload
optimization
and
adequate
surgical
repair
(exclude
valve
dysfunction,
coronary
graft
failure,
LV
outflow
tract
obstruction)
Inability
to
wean
from
CPB
despite
preload
and
pharmacologic
optimization
(MAP
<
70,
Cl
<
2.0
L/min/m
2
,
SvO
2
<
70%,
elevated
[lactate])
HR
70–100
Optimize
preload
High
MAP
70–90
mm
Hg
<
70
mm
Hg
2+
,
K
+
venous
return
and
pump
flow:
100%
–
75%
–
50%
–
25%
–
0
(reservoir,
cell-saver)
1.
Inotropes
±
vasopressors
Or
levosimendan
(or
milrinone)
+
norepinephrine
2.
Stepwise
reduction
of
venous
return
and
pump
flow:
100%
–
75%
–
50%
–
25%
–
0
3.
Cardiac
pacing:
biventricular,
atrioventricular
4.
Inhaled
NO
(PGI
2
)
if
PH,
RV
failure
(NTG,
NPS)
Vasopressors
1.
Norepinephrine,
phenylephrine
2.
Terlipressin
3.
(Methylene
blue)
>
100
beats/min
Arrhythmia
<
70
beats/min
or
conduction
block
>
90
mm
Hg
Step
2
TEE
Ventricular
function
Vasoplegic
syndrome
Ventricular
failure
Appropriate
Impaired
→
Successful
CPB
weaning
Protamine
IV
and
ACT
control
Fig.
54.11
Algorithm
for
weaning
from
cardiopulmonary
bypass
(CPB).
ACT,
Activated
clotting
time;
Hct,
hematocrit;
HR,
heart
rate;
IV,
intravenous;
K
+
,
potassium;
LV,
left
ventricular;
MAP,
mean
arterial
pressure;
Mg
2+
,
magnesium;
NO,
nitric
oxide;
NPS,
sodium
nitroprusside;
NTG,
nitroglycerin;
PGI
2
,
prostacyclin;
PH,
pulmonary
hypertension;
RV,
right
ventricular;
SvO
2
,
mixed
venous
oxygen
saturation;
TEE,
transesophageal
echocardiography.
(From
Licker
M,
Diaper
J,
Cartier
V,
et
al.
Clinical
review:
management
of
weaning
from
cardiopulmonary
bypass.
Ann
Card
Anaesth.
2012;15:206–223.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-101-320.jpg)








































![Anesthesia
and
the
Renal
and
Genitourinary
Systems
1933
Patients
remain
asymptomatic
with
only
biochemical
evi-
dence
of
a
decline
in
GFR
(i.e.,
an
increase
in
serum
concen-
trations
of
urea
and
creatinine).
Further
workup
usually
reveals
other
abnormalities,
such
as
nocturia,
anemia,
loss
of
energy,
decreasing
appetite,
and
abnormalities
in
cal-
cium
and
phosphorus
metabolism.
As
the
GFR
decreases
further,
a
stage
of
severe
renal
insufficiency
begins.
This
stage
is
characterized
by
pro-
found
clinical
manifestations
of
uremia
and
biochemical
abnormalities,
such
as
acidemia;
volume
overload;
and
neurologic,
cardiac,
and
respiratory
manifestations.
At
the
stages
of
mild
and
moderate
renal
insufficiency,
intercur-
rent
clinical
stress
may
compromise
renal
function
further
and
induce
signs
and
symptoms
of
overt
uremia.
When
the
GFR
is
5%
to
10%
of
normal,
it
is
called
end-stage
renal
dis-
ease
(ESRD),
and
continued
survival
without
renal
replace-
ment
therapy
becomes
impossible
(Table
59.3).
Blood
Urea
Nitrogen
The
blood
urea
nitrogen
(BUN)
concentration
is
not
a
direct
correlate
of
reduced
GFR.
BUN
is
influenced
by
nonrenal
variables,
such
as
exercise,
bleeding,
steroids,
and
massive
tissue
breakdown.
The
more
important
factor
is
that
BUN
is
not
elevated
in
kidney
disease
until
the
GFR
is
reduced
to
almost
75%
of
normal.
1
Creatinine
and
Creatinine
Clearance
Measurements
of
creatinine
provide
valuable
information
regarding
general
kidney
function.
Creatinine
in
serum
results
from
turnover
of
muscle
tissue
and
depends
on
daily
dietary
intake
of
protein.
Normal
values
are
0.5
to
1.5
mg/100
mL;
values
of
0.5
to
1
mg/100
mL
are
pres-
ent
during
pregnancy.
Creatinine
is
freely
filtered
at
the
glomerulus,
and
apart
from
an
almost
negligible
increase
in
content
because
of
secretion
in
the
distal
nephron,
it
is
neither
reabsorbed
nor
secreted.
Serum
creatinine
mea-
surements
reflect
glomerular
function
(Fig.
59.4),
4
and
creatinine
clearance
is
a
specific
measure
of
GFR.
Creati-
nine
clearance
can
be
calculated
by
the
following
formula
derived
by
Cockcroft-Gault
that
accounts
for
age-related
decreases
in
GFR,
body
weight,
and
sex:
(
)
[(
)
(
)]
[
(
)
]
This
value
should
be
multiplied
by
0.85
for
women
because
a
lower
fraction
of
body
weight
is
composed
of
muscle.
Because
there
is
such
a
wide
range
in
normal
values,
a
50%
increase
in
serum
creatinine
concentration,
indica-
tive
of
a
50%
reduction
in
GFR,
may
go
undetected
unless
baseline
values
are
known.
In
addition,
excretion
of
drugs
dependent
on
glomerular
filtration
may
be
significantly
decreased
despite
what
might
seem
to
be
only
slightly
ele-
vated
serum
creatinine
values
(1.5-2.5
mg/100
mL).
The
serum
creatinine
concentration
and
clearance
are
better
indicators
of
general
kidney
function
and
GFR
than
are
similar
measurements
of
urea
nitrogen
(Box
59.1).
How-
ever,
there
are
disease
states
in
which
even
the
serum
cre-
atinine
can
be
affected
independent
of
the
GFR
(Table
59.4).
The
main
limitation
of
current
GFR
estimates
is
the
greater
inaccuracy
in
populations
without
known
chronic
kidney
disease
than
in
those
with
the
disease.
Nonetheless,
current
GFR
estimates
facilitate
detection,
evaluation,
and
man-
agement
of
the
disease,
and
they
should
result
in
improved
patient
care
and
better
clinical
outcomes.
5
TABLE
59.3
Clinical
Abnormalities
in
Chronic
Renal
Failure
and
Their
Response
to
Dialysis
and
Erythropoietin
Treatment
Improved
by
Dialysis
Improved
by
Adding
Erythropoietin
Variable
Response
Not
Improved
Develop
After
Dialysis
Therapy
Volume
expansion
and
contraction
Hypernatremia
and
hyponatremia
Hyperkalemia
and
hypokalemia
Metabolic
acidosis
Hyperphosphatemia
Hypocalcemia
Vitamin
D–deficient
osteomalacia
Carbohydrate
intolerance
Hypothermia
Asterixis
Muscular
irritability
Myoclonus
Coma
Congestive
heart
failure
or
pulmonary
edema
Pericarditis
Uremic
lung
Ecchymoses
Uremic
frost
Anorexia
Nausea
and
vomiting
Uremic
fetor
Gastroenteritis
Fatigue
Impaired
mentation
Lethargy
Pallor
Anemia
Bleeding
diathesis
Secondary
hyperparathy-
roidism
Hyperuricemia
Hypertriglyceridemia
Protein-calorie
malnutrition
Headache
Peripheral
neuropathy
Restless
legs
syndrome
Paralysis
Seizures
Myopathy
Arterial
hypertension
Cardiomyopathy
Accelerated
atherosclerosis
Vascular
calcification
Hyperpigmentation
Peptic
ulcer
Gastrointestinal
bleeding
Increased
susceptibility
to
infection
Increased
lipoprotein
level
Decreased
high-density
lipoprotein
level
Impaired
growth
and
development
Infertility
and
sexual
dysfunction
Amenorrhea
Sleep
disorders
Pruritus
Lymphocytopenia
Splenomegaly
and
hypersplenism
Adynamic
osteomalacia
β
2
-Microglobulinemia
Muscle
cramps
Dialysis
dysequilibrium
syndrome
Hypotension
and
arrhythmias
Hepatitis
Idiopathic
ascites
Peritonitis
Leukopenia
Hypocomplementemia](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-142-320.jpg)

![Anesthesia
and
the
Renal
and
Genitourinary
Systems
1937
and
meperidine.
For
the
fentanyl
congeners,
the
clinical
importance
of
renal
failure
is
less
marked.
15
Morphine
is
an
opioid
with
active
metabolites
that
depend
on
renal
clearance
mechanisms
for
elimination.
Morphine
is
principally
metabolized
by
conjugation
in
the
liver,
and
the
water-soluble
glucuronides
(morphine-3-glucuronide
and
morphine-6-glucuronide)
are
excreted
via
the
kidney.
The
kidney
also
plays
a
role
in
the
conjugation
of
morphine,
accounting
for
nearly
40%
of
its
metabolism.
16
Patients
with
renal
failure
can
develop
high
levels
of
morphine-
6-glucuronide
and
life-threatening
respiratory
depression.
In
view
of
these
changes
induced
by
renal
failure,
alterna-
tives
to
morphine
should
be
considered
in
patients
with
severely
altered
renal
clearance
mechanisms.
15
The
clinical
pharmacology
of
meperidine
is
also
signifi-
cantly
altered
by
renal
failure.
Normeperidine,
the
chief
metabolite,
has
analgesic
and
central
nervous
system
(CNS)
excitatory
effects.
Because
the
active
metabolites
are
sub-
ject
to
renal
excretion,
this
potential
CNS
toxicity
secondary
to
normeperidine
accumulation
is
especially
a
concern
in
patients
in
renal
failure.
17
The
clinical
pharmacology
of
the
fentanyl
congeners
is
not
grossly
altered
by
renal
failure,
although
a
decrease
in
plasma
protein
binding
potentially
can
alter
the
free
frac-
tion
of
the
fentanyl
class
of
opioids.
15
As
with
fentanyl,
suf-
entanil
pharmacokinetics
are
not
altered
in
any
consistent
fashion
by
renal
disease,
although
greater
variability
exists
in
the
clearance
and
elimination
half-life
of
sufentanil
when
patients
have
impaired
renal
function.
18
An
increased
clini-
cal
effect
is
likely
with
alfentanil
in
renal
failure
because
of
a
decreased
initial
volume
of
distribution
and
an
increased
free
fraction
of
alfentanil.
19
However,
no
delay
in
recovery
after
alfentanil
administration
should
be
expected.
Neither
the
pharmacokinetics
nor
the
pharmacodynamics
of
remi-
fentanil
are
altered
by
impaired
renal
function.
20
Hydromorphone,
as
the
parent
drug,
does
not
substan-
tially
accumulate
in
hemodialysis
patients.
Conversely,
an
active
metabolite,
hydromorphone-3-glucuronide,
quickly
accumulates
between
dialysis
treatments
but
seems
to
be
effectively
removed
during
hemodialysis.
21
With
careful
monitoring,
hydromorphone
can
be
used
safely
in
patients
who
require
dialysis.
However,
it
should
be
used
with
caution
in
patients
with
a
GFR
less
than
30
mL/min
and
who
have
yet
to
start
dialysis
or
who
have
withdrawn
from
dialysis.
Inhaled
Anesthetics
All
inhaled
anesthetics
are
biotransformed
to
some
extent,
with
the
nonvolatile
products
of
metabolism
eliminated
almost
entirely
by
the
kidney.
22
Reversal
of
the
CNS
effects
of
inhaled
anesthetics
depends
on
pulmonary
excretion;
therefore
impaired
kidney
function
would
not
alter
the
response
to
these
anesthetics.
From
the
viewpoint
of
select-
ing
an
anesthetic
that
would
not
be
harmful
to
patients
with
mild
or
moderate
impairment
of
renal
function,
all
of
the
modern
potent
inhaled
vapor
anesthetics
are
accept-
able.
Fluoride
levels
after
isoflurane
increase
by
only
3
to
5
µM
23
and
by
only
1
to
2
µM
after
halothane
24
;
therefore
these
anesthetics
have
no
nephrotoxic
potential.
Desflurane
and
sevoflurane,
two
newer
inhaled
anesthet-
ics,
are
remarkably
different
from
each
other
with
respect
to
their
molecular
stability
and
biotransformation.
Desflurane
is
highly
stable
and
resists
degradation
by
soda
lime
25
and
the
liver.
The
mean
inorganic
fluoride
concentration
after
1
minimum
alveolar
concentration
(MAC)-hour
exposure
to
desflurane
was
less
than
1
µM.
26
The
safety
of
desflurane
in
renal
failure
patients
has
been
confirmed.
In
addition,
more
sensitive
indices
of
renal
function—urine
retinol-binding
protein
and
β-N-acetylglucosaminidase—showed
no
evi-
dence
of
renal
damage.
Prolonged
exposure
to
desflurane
(7
MAC-hours)
has
been
associated
with
normal
renal
function.
27
Sevoflurane
is
not
very
stable.
Soda
lime
causes
it
to
decompose,
28
and
it
is
biotransformed
by
the
liver.
Plasma
inorganic
fluoride
concentrations
approaching
nephrotoxic
levels
(50
µmol/L)
29
have
been
reported
after
prolonged
inhalation
of
sevoflurane.
However,
no
evidence
of
gross
changes
in
renal
function
has
been
found
in
humans.
30
Data
also
suggest
that
sevoflurane
can
safely
be
delivered
at
fresh
gas
flows
as
low
as
1
L/min
without
significant
pro-
duction
of
a
breakdown
product
named
compound
A
(flu-
oromethyl-2,2-difluoro-1-[trifluoromethyl]
vinyl
ether),
which
is
considered
potentially
nephrotoxic.
31
Inhaled
anesthetics
cause
a
transient
reversible
depres-
sion
in
renal
function.
GFR,
renal
blood
flow,
urine
output,
and
urinary
excretion
of
sodium
are
decreased
(Table
59.8).
Probable
mechanisms
include
loss
of
renal
autoregulation,
activation
of
neurohumoral
factors
(e.g.,
antidiuretic
hor-
mone,
vasopressin,
renin),
and
neuroendocrine
responses.
Although
most
inhaled
anesthetics
have
been
shown
to
reduce
GFR
and
urinary
excretion
of
sodium,
studies
exam-
ining
their
effects
on
renal
blood
flow
have
yielded
con-
flicting
results,
which
can
be
explained
by
differences
in
experimental
methodology.
Data
suggest
that
renal
blood
flow
is
maintained
with
halothane,
isoflurane,
and
desflu-
rane
32,33
but
that
it
is
decreased
with
sevoflurane.
34
Intravenous
Anesthetics
Reversal
of
CNS
effects
after
the
administration
of
ultra-
short-acting
barbiturates
such
as
thiopental
and
metho-
hexital
occurs
as
a
result
of
redistribution,
and
hepatic
metabolism
is
the
sole
route
of
elimination
of
these
drugs.
Thiopental
is
75%
to
85%
bound
to
albumin,
35
the
con-
centration
of
which
may
be
markedly
reduced
in
uremia.
TABLE
59.7
Drugs
Used
or
Encountered
in
Anesthesia
Practice
that
Significantly
Depend
on
Renal
Elimination
Completely
Dependent
Partially
Dependent
Digoxin,
inotropes
(used
fre-
quently;
monitoring
of
blood
levels
indicated
in
chronic
renal
failure)
Intravenous
anesthetics—
barbiturates
Others—aminoglycosides,
van-
comycin,
cephalosporins,
and
penicillins
Muscle
relaxants—pancuronium
Anticholinergics—atropine,
glycopyrrolate
Cholinesterase
inhibitors—
neostigmine,
edrophonium
Others—milrinone,
hydralazine,
cycloserine,
sulfonamides,
and
chlorpropamide](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-144-320.jpg)





























![SECTION IV Adult Subspecialty Management
2104
stasis may result from decreased vascular compliance, a
low-flow state such as congestive heart failure, immobil-
ity, varicose veins, postmenopausal estrogen replacement
therapy, and smoking.2
Myocardium
In the absence of pathology, systolic function typically
remains well preserved throughout life; however, diastolic
dysfunction becomes more common. Age-related myo-
cyte death and reciprocal increases in myocyte size lead to
myocardial thickening and decreased elasticity.3 Chronic
hypertension can further exacerbate cardiac hypertrophy.
Ventricular thickening and stiffening, in turn, impair early
diastolic filling, which falls to 50% of its peak by the age of
80 years.4 In order to maintain cardiac output, geriatric
patients are increasingly dependent on preload and atrial
kick. Conversely, small decreases in circulating blood vol-
ume can lead to inadequate cardiac filling, which can sig-
nificantly decrease cardiac output.
Cardiac output is also limited by a lower maximal heart
rate relative to younger adults3; maximal heart rate can be
estimated as: HR (bpm) = 220 − age (years). In the absence
0%
2%
4%
6%
% of Americans age >70
% of people age >70 (worldwide)
8%
10%
12%
Population percentage age >70 by decade
1975
C
1980 1985 1990 1995 2000 2005 2010 2015
Fig. 65.1 cont’d
Altered intercellular
communication
Genomic instability
Telomere attrition
Epigenetic
alterations
Loss of
proteostasis
Deregulated
nutrient-sensing
Mitochondrial
dysfunction
Cellular
senescence
Stem cell
exhaustion
Fig. 65.2 Molecular, cellular, and organ-level mechanisms of aging. (Redrawn from López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging.
Cell. 2013;153[6]:1194–1217.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-174-320.jpg)


![SECTION IV Adult Subspecialty Management
2110
and palliative care is relatively new; however one study
found that frailty screening in an elderly veterans’ hospi-
tal surgical cohort increased preoperative palliative care
consultation and was associated with a 33% reduction in
180-day mortality.61
POLYPHARMACY
Preoperative assessment of medications is very important;
studies suggest that the incidence of discrepancies between
surgical and anesthesiology assessments are greater than
70%.63 The advent of computerized medical records can
be helpful or harmful as medications which are not deleted
from the record may continue to appear as current. There-
fore medical reconciliation at admission and discharge is
required to assure up-to-date information. Best practice
may include working with pharmacists to review patient
medications for polypharmacy and potential drug inter-
actions and contraindicated medications for older adults.
These include the American Geriatric Society’s Beers Cri-
teria for Potentially Inappropriate Medication Use in Older
Adults.64 The list includes several medications commonly
seen in anesthesia order sets: meperidine, scopolamine,
benzodiazepines (Table 65.4).
DEPRESSION AND ALCOHOL SCREENING
Depression and alcohol abuse each have approximately a
10% incidence in older adults, and each are associated with
a more difficult postoperative course. The former is associ-
ated with greater pain perception and increased need for
postoperative analgesics, and the latter postoperative com-
plications such as pneumonia and sepsis.65 Depression can
be assessed using tools such as the Patient Health Question-
naire -266 which asks:
“In the past 12 months have you ever had a time when
you felt sad, blue, depressed or down for most of the time for
at least 2 weeks?”
“In the past 12 months have you ever had a time lasting
at least 2 weeks when you didn’t care about the things you
usually cared about or when you didn’t enjoy the things
that you usually enjoyed?”
“Yes” to either constitutes a positive screen which needs
further evaluation.
The classic screening tool for alcohol use is the modi-
fied CAGE questionnaire which has been validated for
use in older adults.67,68 The questionnaire consists of four
questions:
□ Have you ever felt you needed to cut down on your
drinking?
□ Have people annoyed you by criticizing your drinking?
□ Have you ever felt guilty about drinking?
□ Have you ever felt you needed a drink first thing in the
morning (Eye Opener) to steady your nerves or get rid of
a hangover?
“Yes” to any question triggers consideration for periop-
erative prophylaxis for withdrawal syndromes, supplemen-
tation of folic acid and thiamine, and consideration of the
need for a detoxification protocol supervised by an addic-
tion specialist.43
CAPACITY/ADVANCED DIRECTIVES/
EXPECTATIONS/SUPPORT
Capacity
In working with older adults, it is important to under-
stand whether they retain the capacity for medical deci-
sion making. Cognition may overlap with capacity but
is not the same thing. Many patients with mild cognitive
TABLE 65.3 Frailty Assessment Tools and Scoring Systems in Current Literature
Frailty Measure Description Clinical Outcome Source
Frailty phenotype Weight loss, grip strength, exhaustion, low physical
activity, and 15 feet walking speed
30 days postoperative complications,
institutionalization, and length of stay
Makary et al.52
Revenig et al.97
Frailty index/deficit
accumulation
30-70 measures of comorbidity, ADL, physical and
neurological exam
Mortality and institutionalization Mitnitski et al.98
Rockwood et al.99
Modified frailty
index
History of diabetes, COPD or pneumonia; congestive
heart failure; myocardial infarction; angina/PCI;
hypertension requiring medication; peripheral
vascular disease; dementia; TIA or CVA; CVA with
neurological deficit; ADL
30-day, 1-year, and 2-year mortality, 30-day
major postoperative
complications
Adams et al.100
Farhat et al.101
Karam et al.102
Obeid et al.103
Patel et al.104
Tsiouris et al.105
Velanovich et al.106
Gait speed 5-m gait ≥6 s Mortality, major postoperative complications,
institutionalization, and length of stay
Afilalo et al.107
Timed up and go TUG ≤10 s; 11-14 s; ≥15 s 1-year mortality Robinson et al.108
Falls 6-month hx of falls 30-day major postoperative complications,
institutionalization, and 30-day readmission
Jones et al.109
Robinson Katz Score, Mini cognition, Charlson Index,
anemia <35%, albumin <3.4, hx of falls
30-day major postoperative complications,
length of stay, 30-day readmission,
6-month postoperative mortality
Robinson et al.110,111
ADL, Activities of daily living; Cog, cognition; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; PCI, percutaneous coronary interven-
tion; TIA, transient ischemic attack; TUG, Timed Up and Go.
(From Amrock LG, Deiner S. The implication of frailty on preoperative risk assessment. Curr Opin Anaesthesiol. 2014;27[3]:330–335.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-177-320.jpg)


![SECTION IV Adult Subspecialty Management
2116
care; the verification process is voluntary and identifies the
presence of resources considered essential for the optimal
care of the injured patient.8 Trauma center levels range
from Level I (a comprehensive regional resource providing
24-hour in-house coverage, referral resource for communi-
ties in nearby regions, leadership in prevention, research,
and more) to Level V (basic emergency department [ED]
facilitiestoimplementadvancedtrauma life support[ATLS],
after-hours activation protocols, limited surgery and criti-
cal care). Level I and Level II centers represent tertiary care
centers; the standards for the provision of clinical care to
injured patients for Level I and Level II trauma centers are
identical. A trauma system is an example of tiered region-
alization because the most seriously injured patients in a
geographical catchment area are cared for at designated
tertiary care trauma centers.7 Over the past three decades,
many studies have demonstrated significantly improved
mortality,9-15 morbidity,16,17 and cost savings17,18 after
establishment of regionalized trauma systems.
THE ROLE OF THE ANESTHESIOLOGIST
At all levels of trauma care, anesthesiologists are uniquely
juxtaposed with the multidisciplinary trauma team, serv-
ing both an administrative role in preparing the operat-
ing room (OR) and allocating resources for resuscitation,
while providing direct patient care through definitive
airway management and advanced resuscitation where
appropriate.19 Anesthesiologists also play a significant
role as intensivists and pain management experts. Trauma
patients represent a significant proportion of all OR cases
handled during night and weekend shifts.20 Regrettably,
very few anesthesiologists in the United States consider
trauma their primary specialty. This is distinct from Euro-
pean practice, where anesthesiologists frequently are found
working in the prehospital environment, as an ED director,
or as leader of a trauma team. The United States model, in
which all anesthesiologists treat trauma patients—but few
do so exclusively—has led to a relative dearth of research,
publication, and education in this field.20,21 This situation
is unfortunate because trauma is a rapidly evolving field of
study that presents unique challenges to the clinician and
one in which improvements in care can have a dramatic
impact on society as a whole.
Anesthesia for trauma patients is different from routine
OR practice. Most urgent cases occur during off-hours,
when the most experienced OR and anesthesia personnel
may not be available. In small hospitals and military and
humanitarian practice, austere conditions may influence
the resources available. Patient information may be limited,
and allergies, genetic abnormalities, and previous surger-
ies may create sudden crises. Patients are frequently intoxi-
cated, with full stomachs and the potential for cervical spine
instability. Simple operations may become complicated,
and specialty surgical and anesthesia equipment may be
required on short notice. Patients often have multiple inju-
ries requiring complex positioning, multiple procedures,
and the need to consider priorities in management. Occult
injuries, such as tension pneumothorax, can manifest at
unexpected times. Fortunately, there does not appear to be
a higher risk for medical liability associated with the pro-
vision of anesthesia for trauma versus nontrauma surgical
anesthesia cases.22 Successful perioperative care of these
patients requires a good understanding of the basics, sup-
plemented by preparation, flexibility,andthe ability to react
quickly to changing circumstances.
This chapter provides an overview of important areas
of trauma care for the anesthesiologist beginning with a
description of the initial approach to an injured patient, fol-
lowed by discussions of emergency airway management,
resuscitation, and care of patients with central nervous
system (CNS) injuries. The needs of orthopedic and recon-
structive surgery patients are outlined and the chapter
concludes with a discussion of postoperative issues for the
anesthesiologist managing the trauma patient.
Prioritizing Trauma Care
PREHOSPITAL TRIAGE
Prehospital triage of the seriously injured trauma patient
begins in the field, and is fraught with difficulty.Estimations
of blood loss are imprecise and classically taught shock clas-
sifications are commonly confounded by extremes of age
and variations in physiological reserve.23 In 2011, the
Centers for Disease Control and Prevention along with the
National Highway Traffic Safety Administration collabo-
rated with the ACS Committee on Trauma to revise previ-
ous field triage decision schemes in order to reduce over
triage of patients with non–life-threatening injuries, and to
help direct patients in most need of lifesaving interventions
to appropriate trauma centers.24 Current guidelines recom-
mend a four-step assessment to assist prehospital providers
with making decisions about which patients are most in
need of transport to a trauma center (Box 66.1).
Physiological Considerations
Systolic blood pressure <90 mm Hg
Glasgow Coma Scale ≤13
Respiratory rate <10 or >29 (or need for ventilatory support)
Anatomical Considerations
Any penetrating injury to the head, neck, torso, and extremities
(proximal to the elbow or knee)
Chest wall instability/deformity
Amputation proximal to the wrist or ankle
Pelvic fracture
Open/depressed skull fracture
Paralysis
Mechanisms of Injury
Death of occupant in same vehicle
Fall from >20 feet
Extrication time >20 min
Special Patient or System Considerations
Age >55 years
Children
Patients on anticoagulants or with bleeding disorders
Burns (to be triaged to designated burn centers)
Pregnancy >20 weeks
BOX 66.1 Four Steps for Assessing Need
for Trauma Center Referral](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-180-320.jpg)








![SECTION IV Adult Subspecialty Management
2134
tailored approach can be accomplished using an algorithm
(Fig. 66.9) to guide specific product selection and limit
exposure to unnecessary blood products.
Additionally, during this time, attention to other physi-
ologic considerations can proceed. For example, hypother-
mia is commonly present on initial presentation. During
phase 1, all attempts should be made to initiate active fluid
and surface warming although it is difficult to fully correct
during a massive resuscitation. During phase 2, additional
measures can be instituted as the focus shifts from control
of hemorrhage to stabilization of all physiologic processes.
Phase 3, Restoration of Physiology
Box 66.7 summarizes endpoints for late resuscitation, and
Fig. 66.10 presents an algorithm for management. Intrave-
nous fluid administration is an integral, mandatory compo-
nent. The adequacy of resuscitation should not be judged
by the presence of normal vital signs, but by restoration of
organ and tissue perfusion. The role of the anesthesiologist-
intensivist is to recognize the presence of ongoing shock
after traumatic hemorrhage and to resuscitate the patient
with the appropriate type and amount of fluids intrave-
nously at the appropriate time.
Late resuscitation begins once bleeding is definitively
controlled by surgery, angiography, or the passage of time.
The goal at this time is to restore normal perfusion to all
organ systems while continuing to support vital functions.
Hypoperfusion caused by hemorrhagic shock triggers a pre-
dictable cascade of biochemical events that will cause phys-
iologic derangements persisting long after adequate blood
flow is restored. The extent of hypoperfusion—the depth
and duration of shock—dictates the magnitude of subse-
quent organ system failure. Unfortunately, traditional vital
sign markers such as arterial blood pressure, heart rate,
and urine output are insensitive to the adequacy of resus-
citation. Occult hypoperfusion syndrome is common in
postoperative trauma patients, particularly young ones.189
This syndrome is characterized by a normal blood pressure
maintained by systemic vasoconstriction, decreased intra-
vascular volume and cardiac output, and organ system
ischemia. The patient will be at frequent risk for MOD if the
hypoperfusion is not promptly corrected.
The search for the optimal endpoints of resuscitation
has led to several different hemodynamic, acid-base, and
regional perfusion targets. Table 66.5 summarizes modali-
ties that are available to gauge the adequacy of resusci-
tation, along with the shortcomings of each technique.
Although the flow of blood to tissue beds is a determinant of
tissue perfusion, pressure should also be an important con-
sideration. The left ventricular stroke work index is a vari-
able that accounts for both flow and pressure. Furthermore,
FFP
Cryoprecipitate
Platelets
Aminocaproic
acid
MCF exTEM 45 mm
and
MCF fibTEM 10 mm
MCF fibTEM 8 mm
CT inTEM 230 sec
Diffuse bleeding
Normal results
Pathologic
results
ROTEM
Consider limitations
of ROTEM
Surgical hemostasis
ML exTEM 15%
Fig. 66.9 Example of a rotation thromboelastometry (ROTEM) treatment algorithm for use in trauma. CT, Clotting time; FFP, fresh frozen plasma; MCF,
maximum clot firmness; ML, maximum lysis. (Courtesy of San Francisco General Hospital and Trauma Center. (From Steurer M, Chang T, Lancman B. Anesthe-
sia for trauma. In: Pardo M, Miller RD, eds. Basics of Anesthesia. 7th ed. Philadelphia: Elsevier; 2018:724 [Chapter 42].)
Maintain systolic blood pressure higher than 110 mm Hg
Maintain hematocrit above individual transfusion threshold
Normalize coagulation status
Normalize electrolyte balance
Normalize body temperature
Restore normal urine output
Maximize cardiac output by invasive or noninvasive measurement
Reverse systemic acidosis
Document decrease in lactate to normal range
BOX 66.7 Goals for Late Resuscitation
Fluid administration should be continued until adequate systemic perfu-
sion is restored.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-189-320.jpg)






















![SECTION IV Adult Subspecialty Management
2278
than if the patient were recovering in the hospital envi-
ronment, allowing earlier detection and increased safety.
Daily assessment of various recovery parameters at home
using a smartphone-based app was popular with patients
and also appeared to improve quality of recovery, probably
by providing additional reassurance.488 The system could
also be used to request contact from a nurse and was found
to be more cost-effective than other forms of postdischarge
contact,489 although others found a simple telephone call,
which would be cheaper still, was similarly effective.490
Follow-Up and Outcome Measures
The ASA has developed a set of outcome indicators spe-
cifically for ambulatory and office-based surgery and
anesthesia.491 These include outcome events of interest
for days 1, 14, and 30 and ongoing continuous quality
indicators. The IAAS492 has developed a series of indica-
tors (Table 72.8) useful in the evaluation of the overall
success of organizational performance, and these mirror
the advice from other national specialist societies.46,493
Lemos and Barros494 further defined the value of outcome
reporting in a range of domains subdivided into clinical,
organizational, social, and economic factors that allows
the reporting of both individual and institutional perfor-
mance (Table 72.9). Return-mail questionnaires can be
used for patient follow-up after ambulatory surgery to
help identify common sequelae that ambulatory patients
should realistically expect to experience.391 No matter
how data are collected, it is important that information
from quality indicators is fed back in an effective way to
individual clinicians and clinical units to support continu-
ous improvement.495
Adverse Effects After Ambulatory Surgery
Minor adverse events are common after ambulatory sur-
gery and anesthesia (86%).391 Drowsiness is the most com-
mon effect persisting after discharge (62%), and aches and
sore throat are common in intubated patients (47% and
49%, respectively). Headache (25%) and dizziness (20%)
also occur, but nausea and vomiting after discharge are less
common (17% and 7%, respectively). Patients may take 2
to 3 days before they are able to resume their usual activi-
ties.391 Information about these known side effects should
be incorporated into the preoperative patient education
and, in the United States, may be incorporated in a separate
consent for anesthesia.
Low-Risk Patients
(all those not in high-
risk groups)
May be discharged.
Advise to return if no
voiding within 12 hours
Ask patient to void when
comfortably full and ready
or after maximum of 4 hours
Observe
further
Unable to void
when ready for
discharge
High-Risk Patients
Urology, urogynecology, inguinal or perianal surgery, age >70
years, history of voiding difficulty or incontinence,
spinal anesthesia using >7 mg bupivacaine
Check bladder
volume using
ultrasound
> 400 mL
< 400 mL
Discharge with
catheter; trial without
catheter in 2 days
Voids
Check residual
volume using
ultrasound
< 150 mL 150–400 mL
> 400 mL
Fig. 72.5 Flowchart illustrating the management of patients who fail to void urine after ambulatory surgery. (Reproduced from British Association of Day
Surgery. Spinal anaesthesia for day surgery patients. London [available from http://www.bads.co.uk]; 2010. With permission.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-212-320.jpg)



![SECTION IV • Adult Subspecialty Management
2318
or 4 mg every 12 hours) has also been shown to be benefi-
cial,49,101 but remains a second-line chemoprophylaxis.100
Individual risk is highly variable based on both personal
(e.g., previous history of AMS or High-Altitude Cerebral
Edema [HACE]) and environmental (e.g., rate of ascent,
highest altitude attained) factors. Prescribing of prophylac-
tic medications and indeed any recommendations regard-
ing individuals should consider this personalized risk.
When treating AMS, because of the nonspecific nature
of the symptoms, consideration must be given to other
causes. There may be more severe altitude patholo-
gies such as HACE, or common conditions related to the
nature of much travel to altitude such as dehydration,
exhaustion, hypothermia, hypoglycemia, or infection.100
In cases where AMS is diagnosed, then the single most
efficacious treatment is descent. This may present other
dangers, given the terrain often found in the high alti-
tude environment; however, in severe cases, descent until
resolution of symptoms (which typically occurs after a
descent of as little as 300 m) remains the gold standard
treatment.100 Measures aimed at correcting hypobaric
hypoxia, such as supplemental oxygen and hyperbaric
chambers, can offer possible alternatives to descent but
in a remote high-altitude setting these treatments present
marked logistical challenges, may only offer temporary
benefit, and also carry their own risks.100,102 Dexametha-
sone has been shown to be an effective treatment,102-104
but it does not aid acclimatization and further ascent is not
recommended until the patient is asymptomatic without
dexamethasone.
HIGH-ALTITUDE CEREBRAL EDEMA
HACE is a severe, life-threatening pathology. It is rare, with
a prevalence of 0.28% to 1%.4,105 Because of its rarity,
there is limited systematic evidence regarding risk factors.81
However, (not yet conclusively proven) HACE is now con-
sidered to be a severe form of AMS and may share the same
pathophysiology and risk factors.89,93,106 It is important to
note though that while AMS may rapidly progress to HACE
if left untreated, HACE may also present suddenly without
any preceding symptoms of AMS.
Both animal studies and postmortem examinations have
demonstrated gross edema in HACE sufferers,107,108 with
magnetic resonance imaging (MRI) studies suggesting
this is likely vasogenic in nature.109 In contrast to AMS,
in HACE there is evidence of microhemorrhage, primarily
within the corpus callosum. The presence of microhemor-
rhage may indicate venous obstruction as a key pathologic
process, which may differentiate AMS from HACE.93
HACE may be clinically differentiated from AMS by the
presence of ataxia, confusion, psychiatric changes, or
altered consciousness that may progress rapidly to coma
and death.81,89,93,110 Investigations offer limited benefit on
acute presentation; however, lumbar puncture may reveal
elevated ICP,111 whereas MRI and computed tomography
may reveal changes associated with edema.109
Prevention of HACE should be guided by the same prin-
ciples as AMS, with slow ascent, preacclimatization, and
pharmacologic strategies as previously described.100 The
diagnosis should consider other possible causes for the
observed presentation, but it is important to remember
the mantra that any illness at altitude should be treated
as altitude-related until proven otherwise. When HACE is
diagnosed, its severity should not be underestimated and
promptmanagementisessential.Immediateactionsinclude
supplemental oxygen (aiming for an SpO2 > 90%), admin-
istration of dexamethasone (initial dose of 8 mg by mouth,
or intravenous or intramuscular injections; and immedi-
ately followed by a dose of 4 mg every 6 hours) and, where
logistically possible, descent.81,100 If descent is not possible,
and the airway is adequately protected, then a hyperbaric
chamber is an acceptable temporary alternative.81,93
High-Altitude Pulmonary Edema
HAPE is a form of noncardiogenic pulmonary edema that
occurs in unacclimatized individuals on ascent to altitude
(>2500m).81 TheconditionwasfirstdescribedinSouthAmer-
ican lowlanders on ascent to altitude in 1955,112 and subse-
quently in 1960 by two separate American physicians.113,114
The condition generally occurs within 2 to 5 days
of ascent and is more common with greater altitude. It
remains relatively uncommon under 3000 m and after
more than 1 week at altitude.115,116 As with many alti-
tude pathologies, the rate of ascent, altitude, and individual
TABLE 74.1 Lake Louise Consensus on Acute Mountain
Sickness 2018
2018 Lake Louise Acute Mountain Sickness Score
Symptom Description Score
Headache
None at all 0
A mild headache 1
Moderate headache 2
Severe headache, incapacitating 3
Gastrointestinal symptoms
Good appetite 0
Poor appetite or nausea 1
Moderate nausea or vomiting 2
Severe nausea and vomiting, incapacitating 3
Fatigue and/or weakness
Not tired or weak 0
Mild fatigue/weakness 1
Moderate fatigue/weakness 2
Severe fatigue/weakness, incapacitating 3
Dizziness/light-headedness
No dizziness/light-headedness 0
Mild dizziness/light-headedness 1
Moderate dizziness/light-headedness 2
Severe dizziness/light-headedness,
incapacitating
3
AMS Clinical Functional Score
Overall, if you had AMS symptoms, how did they affect your activities?
Not at all 0
Symptoms present, but did not force any
change in activity or itinerary
1
My symptoms forced me to stop the ascent
or to go down on my own power
2
Had to be evacuated to a lower altitude 3
From Baillie JK. Lake Louise Consensus on Acute Mountain Sickness 2018. In:
altitude.org. Apr 2019 [cited 16 Apr 2019]. Available: http://www.altitude.org/
lake_louise_AMS_score_2018.php](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-216-320.jpg)









![Transfusion guidelines for nonneonatal pediatric
patients are similar to those for adults.235 Some precau-
tions, however, should be considered, especially in case
of massive transfusion. When caring for children, the
emphasis should be on blood volume and percentage loss
of blood volume, rather than specific units of blood, since
a unit of blood may constitute several blood volumes in a
preterm infant, but only a fraction of the blood volume of a
robust teenager. These considerations govern the calcula-
tion of the maximal allowable blood loss (MABL) result-
ing in an acceptable hematocrit. MABL takes into account
the effect of patient age, weight, and starting hematocrit
on blood volume. In general, blood volume is approxi-
mately 100 to 120 mL/kg for a preterm infant, 90 mL/kg
for a full-term infant, 70 to 80 mL/kg for a child 3 to 12
months old, and 70 mL/kg for a child older than 1 year of
age. These volumes are merely estimates of blood volume.
The individual child’s blood volume is calculated by simple
proportion by multiplying the child’s weight by the esti-
mated blood volume (EBV) per kilogram. Although several
formulas are available, a simple relationship is easiest to
remember:
( )
Thus if a 3-year-old child weighs 15 kg and has a starting
hematocrit of 38% and if clinical judgment estimates the
desired postoperative hematocrit to be 25%, then the calcu-
lation would be as follows:
[( ) ( )]
di
ox
of
an
in
sio
fo
cl
al
di
ta
gu
th
tw
th
m
1
no
of
bl
pr
tia
ge
gi
ex
PT
fo
na
re](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-226-320.jpg)




![Drugs
Inhaled anesthetic drugs
Local anesthetics (lidocaine)
Cardiac antiarrhythmics (procainamide)
Antibiotics (polymyxins, aminoglycosides, lincosamines [clindamycin],
metronidazole [Flagyl], tetracyclines)
Corticosteroid agents
Calcium channel blockers
Dantrolene
Metabolic and Physiologic States
Hypermagnesemia
Hypocalcemia
Hypothermia
Respiratory acidosis
Hepatic or renal failure
Myasthenia syndromes
Excessive dose of succinylcholine
Reduced plasma cholinesterase activity
Decreased levels
□ Extremes of age (newborn, old age)
□ Disease states (hepatic disease, uremia, malnutrition, plas-
mapheresis)
□ Hormonal changes
□ Pregnancy
□ Contraceptives
□ Glucocorticoids
Inhibited activity
□ Irreversible (echothiophate)
□ Reversible (edrophonium, neostigmine, pyridostigmine)
Genetic variant (atypical plasma cholinesterase)
BOX 80.2 Factors Contributing to
Prolonged Nondepolarizing Neuromuscular
Blockade](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-231-320.jpg)






![83 • Critical Care Anesthesiology 2665
achieved in certain types of cancer (melanoma, leukemias,
lymphomas) for which immunotherapy is now being used
as a standard of care.
Besides the desired antitumor effects, immunotherapy
can also trigger unique toxicities resulting from excessive
immune system activation. These may be restricted to cer-
tain organs (colitis, pneumonitis) or be a systemic inflam-
matory response (cytokine release syndrome [CRS]). When
severe, immunotherapy toxicities can be life-threatening
and their management may require admission to the ICU
for continuous monitoring andsupportive care. Intensivists
thus increasingly find themselves leading and coordinating
multidisciplinary care for complex oncologic patients.
IMMUNE CHECKPOINT INHIBITORS
Immunecheckpointinhibitorsarethemostcommonlyadmin-
isteredformofcancerimmunotherapy.Theseagentsenhance
antitumor activity of the patient’s own T cells by blocking
certain T-cell inhibitory signals. FDA-approved inhibitors of
PD-1 (pembrolizumab, nivolumab), PD-L1 (atezolizumab,
avelumab, durvalumab), and CTLA-4 (ipilimumab) are used
in treatments of various solid tumors including melanoma,
non-small-cell lung cancer, head and neck squamous can-
cers, and renal cell carcinoma. These agents can produce
a unique set of side effects termed immune-related adverse
events, whichmay bedermatologic, gastrointestinal, hepatic,
endocrine, pulmonary, cardiac, or neurologic inflammatory
complications. These side effects typically manifest weeks to
months after initiation of the therapy.
The mainstay of the management in cases of moder-
ate- and high-grade toxicity is the interruption of the
checkpoint inhibitor and administration of corticosteroids.
Tumor necrosis factor-α antagonists have been used in
cases refractory to steroids. ICU monitoring and supportive
care may be required in cases of severe pneumonitis (oxy-
gen therapy, mechanical ventilation), myocarditis (inotro-
pic support, antiarrhythmics), severe enterocolitis (fluid
and electrolyte repletion), liver failure, and adrenal insuf-
ficiency. Echocardiography should be performed in patients
with new onset dyspnea, pulmonary edema, or hypoten-
sion. Patients receiving prolonged immunosuppression
for the treatment of checkpoint inhibitor toxicity are at an
especially high risk of infectious complications (opportunis-
tic infections, sepsis).200
CHIMERIC ANTIGEN RECEPTOR T CELLS
CAR T cells are genetically engineered T cells that have the
ability to bind to target tumor cells, undergo supraphysi-
ologic activation, and cause tumor cell lysis. Upon comple-
tion of an ex vivo manufacturing process, CAR T cells are
infused into patients and patients typically remain moni-
tored for several days in the hospital setting. Currently,
CAR T cells are used in the treatment of certain relapsed/
refractory hematologic malignancies (leukemias, lympho-
mas, multiple myeloma) but clinical trials in patients with
solid tumors are also ongoing. Two CD19-directed CAR
T-cell products for treatment of B-cell malignancies (non-
Hodgkin lymphoma, acute lymphoblastic leukemia) have
been FDA approved and cases of complete disease remission
have been reported.
However, CAR T-cell therapy is frequently associated
with toxicities that can range from minor (fatigue, fever,
myalgias) to life-threatening (shock, multiple organ dys-
function). These toxicities are primarily driven by proin-
flammatory cytokines (IL-6, IFNγ) that are released upon
CAR T cell–tumor cell interaction. Of importance to ICU
clinicians are severe forms of CRS and neurotoxicity, which
require ICU monitoring and management. CRS typically
manifests within several days after CAR T-cell infusion by a
spectrum of fever, tachycardia, hypotension, or respiratory
insufficiency. The onset of neurotoxicity (encephalopathy,
aphasia, or seizures) can be delayed and is not necessarily
preceded by clinically significant CRS. Fatal cases of diffuse
cerebral edema have been reported.
The mainstay of the therapy of severe forms of CRS is the
administration of antiinflammatory agents (IL-6 antago-
nists, corticosteroids) in conjunction with individualized
supportive care (fluid resuscitation, vasopressors, mechani-
cal ventilation, renal replacement therapy). Neurotoxicity
is treated with anticonvulsants and corticosteroids. It is cru-
cial that other precipitating factors of neurologic, hemody-
namic, or respiratory decline that are common in patients
with advanced cancer are always actively excluded. Sepsis,
which often resembles CRS, is a frequent cause of morbidity
and mortality in this patient population.201,202
Point-of-Care Ultrasound in
Critical Care
As a result of improving technology, decreasing cost, and
widening availability, the use of point-of-care ultrasound
(POCUS) has taken an increasing role in the practice of
critical care. The uses of ultrasound in the critically ill are
manifold, from vascular access to cardiopulmonary evalu-
ation. POCUS allows for rapid and repeated assessments of
the critically ill patient atthe bedside to augment traditional
physical examination and physiologic monitors. Because
techniques and technologies continue to evolve, there are
a multitude of published protocols. Although the exact pro-
tocol used is of less significance, the specific skills and appli-
cations of ultrasound will likely become a core competency
in the ICU.203,204 Ultimately, ultrasound is another tool in
the armamentarium of the intensivist that requires under-
standing of physiology and expert clinical decision making
for safe and beneficial use.
VASCULAR ULTRASOUND
Vascular cannulation is a vital skill in the ICU. Ultrasound
guidance can be used before the procedure to confirm anat-
omy and vessel patency or, preferentially, it can be used in
real time during the procedure. There are different tech-
niques, the two most common being out-of-plane needle
insertion with short axis view of the vessel, or in-plane nee-
dle insertion with a long axis view of the vessel. Ultrasound
can be used for cannulating central venous structures,
arteries, or even peripheral veins.
Central venous access is one of the most common proce-
dures in the ICU. The use of ultrasound imaging to assist
catheter insertion is a Grade 1B recommendation by
the SCCM guidelines.203,205 For the cannulation of the](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-238-320.jpg)








![Cardiopulmonary Resuscitation and Advanced Cardiac Life Support 2729
TABLE 86.2 Summary of Medications Used for Supraventricular Tachycardia
Drug Characteristics Indication(s) Dosing Side Effects
Precautions or Special
Considerations
Adenosine Endogenous purine
nucleoside; briefly
depresses sinus
node rate and AV
node conduction;
vasodilator
□ Stable, narrow-complex
regular tachycardias
□ Unstable narrow-
complex regular
tachycardias while
preparations are
made for electrical
cardioversion
□ Stable, regular,
monomorphic, wide-
complex tachycardia
as a therapeutic and
diagnostic maneuver
6 mg IV as a rapid IV push followed
by a 20 mL saline flush; repeat if
required as 12 mg IV push
Hypotension,
broncho-
spasm, chest
discomfort
Contraindicated in
patients with asthma;
may precipitate atrial
fibrillation, which
may be very rapid in
patients with WPW;
thus a defibrillator
should be readily
available; reduce dose
in post–cardiac trans-
plant patients, those
taking dipyridamole
or carbamazepine and
when administered
via a central vein
Diltiazem,
Verapamil
Non-dihydropyridine
calcium channel
blockers; slow AV
node conduction
and increase AV
node refractoriness;
vasodilators,
negative inotropes
□ Stable, narrow-
complex tachycardias
if rhythm remains
uncontrolled or
unconverted by
adenosine or vagal
maneuvers or if SVT
is recurrent
□ Control ventricular
rate in patients with
atrial fibrillation or
atrial flutter
Diltiazem: Initial dose 15-20 mg
(0.25 mg/kg) IV over 2 min;
additional 20-25 mg
(0.35 mg/kg) IV in 15 min if
needed; 5-15 mg/h IV
maintenance infusion (titrated
to AF heart rate if given for rate
control)
Verapamil: Initial dose
2.5-5 mg IV given over 2 min;
may repeat as 5-10 mg every
15-30 min to total dose of 20-30
mg
Hypotension,
bradycardia,
precipita-
tion of heart
failure
Should only be given
to patients with
narrow-complex
tachycardias (regular
or irregular). Avoid in
patients with heart
failure and preex-
cited AF or flutter or
rhythms consistent
with VT
Atenolol,
Esmolol,
Metoprolol,
Propranolol
β-Blockers; reduce
effects of circulat-
ing catecholamines;
reduce heart rate,
AV node conduction
and blood pressure;
negative inotropes
□ Stable, narrow-
complex tachycardias
if rhythm remains
uncontrolled or
unconverted by
adenosine or vagal
maneuvers or if SVT
is recurrent
□ Control ventricular
rate in patients with
atrial fibrillation or
atrial flutter
□ Certain forms of
polymorphic VT
(associated with
acute ischemia,
familial LQTS,
catecholaminergic)
Atenolol (β1 specific blocker) 5 mg
IV over 5 min; repeat 5 mg in 10
min if arrhythmia persists or recurs
Esmolol (β1 specific blocker with
2- to 9-min half-life) IV loading
dose 500 mcg/kg (0.5 mg/kg)
over 1 min, followed by an
infusion of 50 mcg/kg per min
(0.05 mg/kg/min); if response is
inadequate, infuse second
loading bolus of 0.5 mg/kg over
1 min and increase maintenance
infusion to 100 mcg/kg (0.1 mg/
kg) per min; increment; increase
in this manner if required to
maximum infusion rate of 300
mcg/kg [0.3 mg/kg] per min
Metoprolol (β1 specific blocker) 5 mg
over 1-2 min repeated as required
every 5 min to maximum dose of
15 mg
Propranolol (nonselective β-blocker)
0.5-1 mg over 1 min, repeated
up to a total dose of 0.1 mg/kg if
required
Hypotension,
bradycardia,
precipita-
tion of heart
failure
Avoid in patients with
asthma, obstruc-
tive airway disease,
decompensated
heart failure and
pre-excited atrial
fibrillation or flutter
Procain-
amide
Sodium and potas-
sium channel
blocker
□ Preexcited atrial
fibrillation
20-50 mg/min until arrhythmia
suppressed, hypotension ensues,
or QRS prolonged by 50%, or total
cumulative dose of 17 mg/kg; or
100 mg every 5 min until arrhyth-
mia is controlled or other condi-
tions described above are met
Bradycardia,
hypoten-
sion,
torsades de
pointes
Avoid in patients with
QT prolongation and
CHF
Amiodarone Multichannel blocker
(sodium, potassium,
calcium channel,
and noncompetitive
α/β-blocker)
□ Stable irregular narrow-
complex tachycardia
(atrial fibrillation)
□ Stable regular narrow-
complex tachycardia
□ To control rapid
ventricular rate due to
accessory pathway con-
duction in pre-excited
atrial arrhythmias
150 mg given over 10 min and
repeated if necessary, followed
by a 1 mg/min infusion for 6 h,
followed by 0.5 mg/min.
Total dose over 24 h should not
exceed 2.2 g.
Bradycardia,
hypoten-
sion, phle-
bitis
Continued](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-247-320.jpg)

![Cardiopulmonary Resuscitation and Advanced Cardiac Life Support 2731
Fig. 86.8 2015 American Heart Association Acute Coronary Syndrome Algorithm. ABC, Airway, breathing, circulation; CPR, cardiopulmonary resuscita-
tion;EMS, emergency medical services; IV, intravenous. (From O’Connor RE, Al Ali AS, Brady WJ, et al. Part 9: Acute Coronary Syndromes: 2015 American Heart
Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132[18 suppl 2]:S483–S500. https://
eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-9-acute-coronary-syndromes/.)](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-249-320.jpg)









![Based on script templates provided by Dieckmann and Rall (https://
www.inpass.de/downloadshtml/downloadscenarioscript/), the
key information a scenario script should provide includes:
Scenario name (quick reference)
Major medical challenge of scenario
Major cockpit resource management (CRM) challenge of sce-
nario
Learning goals (medical and [!] CRM)
Brief narrative description of scenario
Staffing (instructor/simulation team and participants)
Case briefing (all participants together or separate briefings for
different teams)
Simulator setup and mannequin preparation
Props needed
Scenario “lifesavers”259 for both excellent and bad performance
of participants
Usually, the scenario script contains (1) a summary sheet with
brief notes of the above mentioned topics and (2) a more detailed
description of the scenario in regard to those questions on several
other pages. The proper use of scenario scripts is a regular part of
many instructor training courses.
BOX 7.5 Key Considerations for Simulation
Scenario Scripts](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-259-320.jpg)




![Adaptive
Immunity
↓
Splenic
and
thymic
weight
(rodents)
↓
T
cell
viability
and
proliferative
response
↓
T-helper
cell
function
↓
CD4/CD8
population
in vivo
↓
IL1β,
IL-2,
TNF-α,
and
IFN-γ
(mouse
splenocytes)
↓
Th1/Th2
ratio
of
T-helper
cell
population
(PBMCs)
↓
NK
cell
activity
↓
Primary
antibody
response
(B
cells)
↓
B
cells
mitogenic
response
to
bacterial
LPS
↓
Macrophage
activity
↓
TGF-β1
and
IL-10
(antiinflammatory
cytokines)
↑
T
cell
apoptosis
(NF-κβ
and
AP-1/NFAT
pathways)
Inhibition
of
CD3/28
mAb
induced
IL-2
transcripts
Innate
Immunity
↓
Number
of
macrophages
available
to
fight
infections
↓
Leucocyte
migration
↓
Peritoneal
macrophages
phagocytosis
↓
Respiratory
burst
activity
and
chemotaxis
Inhibition
of
Fc
γ
receptor
mediated
phagocytosis
↓
Superoxide
production
from
neutrophils
and
macrophages
Alteration
of
IL-8
induced
neutrophil
chemotaxis
↓
Neutrophil
cytokines
involved
in
wound
healing
↑
Apoptosis
of
macrophages
impairing
host
defense
barrier
↓
Leucocytes
endothelial
adhesion
(intracellular
adhesion
mol-
ecules
expression)
Neuroendocrine
System
↑
Growth
hormone,
prolactin,
and
thyroid
stimulating
hormone
secretion
in
humans
May
affect
the
function
of
the
HPA
axis
(ACTH
and
CRH)
with
risk
of
adrenal
insufficiency
↓
Sex
hormones
[LH
and
testosterone
(hypogonadism)],
oxytocin,
and
estradiol
BOX
24.4
Opioid
Effects
on
Immunity
From
Al-Hashimi
M,
Scott
SW,
Thompson
JP,
Lambert
DG.
Opioids
and
immune
modulation:
more
questions
than
answers.
Br
J
Anaesth.
2013;111:80–88.
ACTH,
Adrenocorticotropic
hormone;
AP-1,
activator
protein
1;
CRH,
cor-
ticotropin
releasing
hormone;
Fc,
fragment
crystallizable
region;
HPA,
hypothalamic
pituitary
adrenal
axis;
IL,
interleukin;
IFN-γ,
interferon-
gamma;
LH,
luteinizing
hormone;
LPS,
lipopolysaccharides;
NF-κβ,
nuclear
factor
kappa
beta;
NFAT,
nuclear
factor
of
activated
T-cells;
NK,
natural
killer;
PBMC,
peripheral
blood
mononuclear
cell;
TGF-β,
trans-
forming
growth
factor
beta;
TNF-α,
tumor
necrosis
factor-alpha.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-264-320.jpg)

![Quantitative Monitoring Used (e.g., Acceleromyography)
1. TOF count of 1 or no TOF response—delay reversal until neuro-
muscular recovery is more complete (TOF count of 2 or greater).
2. TOF count of 2 or 3—administer doses of anticholinesterases
(neostigmine [70 µg/kg], edrophonium [1.0–1.5 mg/kg], or pyri-
dostigmine [350 µg/kg]). Extubate when the adductor pollicis
TOF ratio has reached 0.90.
3. TOF ratio ≥ 0.40—administer moderate pharmacologic reversal
doses of anticholinesterases (neostigmine [40–50 µg/kg], edro-
phonium [0.5 mg/kg], or pyridostigmine [200 µg/kg]). Extubate
when the adductor pollicis TOF ratio has reached 0.90.
4. TOF ratio between 0.40 and 0.70—administer pharmacologic
reversal, consider a low dose of neostigmine (20 µg/kg).
5. TOF ratio > 0.70—avoid anticholinesterase reversal; risk of
anticholinesterase-induced muscle weakness if given.
Qualitative Monitoring Used (Peripheral Nerve Stimulator)
1. TOF count of 1 or no TOF response—delay reversal until neuro-
muscular recovery is detectable (TOF count of 2 or greater)
2. TOF count of 2 or 3 at the end of surgery—administer anticho-
linesterases (neostigmine [70 µg/kg], edrophonium [1.0–1.5 mg/
kg], or pyridostigmine [350 µg/kg]). Allow at least 15-30 minutes
before tracheal extubation is performed.
3. TOF count of 4 with observable fade at the end of surgery (likely
adductor pollicis TOF ratio < 0.40)—administer anticholinester-
ases (neostigmine [40–50 µg/kg], edrophonium [0.5 mg/kg], or
pyridostigmine [200 µg/kg]). Allow at least 10–15 minutes before
tracheal extubation is performed.
4. TOF count of 4 with no perceived fade at the end of surgery
(likely adductor pollicis TOF ratio ≥ 0.40)—administer pharmaco-
logic reversal, consider a low dose of neostigmine (20 µg/kg).
No Neuromuscular Monitoring Used
1. Anticholinesterases should be considered. Spontaneous recov-
ery of neuromuscular function may require several hours in a
significant percentage of patients, even after a single intubating
dose of an intermediate-acting NMBD.
2. Anticholinesterases should not be given until some evidence of
recovery of muscle strength is observed since administration of
an anticholinesterase during deep levels of paralysis may delay
neuromuscular recovery.
3. Decisions relating to the use or avoidance of anticholinester-
ases should not be based upon clinical tests of muscle strength
(5-second head lift). Many patients can perform these tests even
in the presence of profound neuromuscular blockade (TOF ratio
< 0.50). Other muscle groups may be significantly impaired
(pharyngeal muscles) at the time when patients can successfully
perform these tests.
BOX 28.2 Clinical Management Strategies to Reduce the Risk of Residual Neuromuscular Blockade
When Anticholinesterase Reversal Agents Are Used
, Neuromuscular blocking drug; , train-of-four.
Modified from Brull SJ, Murphy GS. Residual neuromuscular block. Lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth
Analg. 2010;111:129–140.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-266-320.jpg)
![SECTION
III
•
Anesthesia
Management
924
weight.
Body
mass
index
(BMI),
which
is
calculated
based
on
height
and
weight,
is
more
informative
than
weight
alone
in
establishing
obesity.
A
scheme
for
clas-
sifying
children
and
adults
based
on
BMI
is
presented
in
Table
31.3.
Information
pertaining
to
BMI
can
help
identify
individuals
at
risk
for
difficulties
with
airway
management,
and
some
chronic
diseases
(e.g.,
heart
dis-
ease,
diabetes
mellitus,
obstructive
sleep
apnea
[OSA]).
An
ideal
body
weight
should
also
be
calculated,
47
using
available
formulae
such
as
the
Devine
equation.
48
Infor-
mation
on
ideal
body
weight
can
better
inform
dose
selection
for
some
anesthesia-related
medications,
and
settings
for
positive
pressure
ventilation.
Readily
avail-
able
online
calculators
can
be
used
to
quickly
determine
both
BMI
and
ideal
body
weight.
Patients
often
have
increased
arterial
blood
pressure
during
the
preopera-
tive
visit,
even
without
a
prior
history
of
hypertension.
This
finding
may
be
caused
by
anxiety,
or
patients
hav-
ing
forgotten
to
take
their
usual
dose
of
antihypertensive
medication.
Thus,
a
single
reading
during
the
preopera-
tive
evaluation
may
not
reflect
the
patient’s
usual
blood
pressure
control.
Repeating
the
blood
pressure
measure-
ment
or
obtaining
previous
readings,
either
by
obtaining
medical
records
(including
prior
ambulatory
blood
pres-
sure
testing)
or
asking
patients
about
their
“usual”
blood
pressure
measurements
are
informative.
Ideally,
the
referral
documentation
from
the
patient’s
primary
care
physician
or
surgeon
should
include
information
on
the
patient’s
usual
blood
pressure
readings.
49
From
an
anesthesiologist’s
perspective,
inspection
of
the
airway
may
be
the
most
important
component
of
the
physical
examination
(see
Chapter
44).
The
components
of
the
airway
examination
are
presented
in
Box
31.1.
50
TABLE
31.2
Duke
Activity
Specific
Index
questionnaire
Can
You
Points
1.
Take
care
of
yourself,
that
is,
eat
dress,
bathe,
or
use
the
toilet?
2.75
2.
Walk
indoors,
such
as
around
your
house?
1.75
3.
Walk
200
yards
on
level
ground?
2.75
4.
Climb
a
flight
of
stairs
or
walk
up
a
hill?
5.50
5.
Run
a
short
distance?
8.00
6.
Do
light
work
around
the
house
like
dusting
or
washing
dishes?
2.70
7.
Do
moderate
work
around
the
house
like
vacuuming,
sweeping
floors,
or
carrying
groceries?
3.50
8.
Do
heavy
work
around
the
house
like
scrubbing
floors
or
lifting
or
moving
heavy
furniture?
8.00
9.
Do
yard
work
like
raking
leaves,
weeding,
or
pushing
a
power
mower?
4.50
10.
Have
sexual
relations?
5.25
11.
Participate
in
moderate
rec-
reational
activities
like
golf,
bowling,
dancing,
doubles
tennis,
or
throwing
a
ball?
6.00
12.
Participate
in
strenuous
sports
like
swimming,
singles
tennis,
football,
basketball,
or
skiing?
7.50
Total
score:
From
Hlatky
MA,
Boineau
RE,
Higginbotham
MB,
et
al.
A
brief
self-adminis-
tered
questionnaire
to
determine
functional
capacity
(the
Duke
Activity
Status
Index).
Am
J
Cardiol.
1989;64:651–654.
TABLE
31.3
Classification
Scheme
for
Body
Mass
Index
Body
Mass
Index
Weight
Status
ADULTS
OVER
20
YEARS
OLD
BMI
<
18.5
Underweight
BMI
18.5–24.9
Normal
BMI
25.0–29.9
Overweight
BMI
30.0
and
above
Obese
FOR
CHILDREN
AND
TEENS
BMI-for-age
<
5th
percentile
Underweight
BMI-for-age
5th
percentile
to
<
85th
percentile
Normal
BMI-for-age
85th
percentile
to
<
95th
percentile
At
risk
of
overweight
BMI-for-age
≥
95th
percentile
Overweight
BMI,
Body
mass
index.
From
Centers
for
Disease
Control
and
Prevention.
http://www.cdc.gov.
Length
of
upper
incisors
(concerning
if
relatively
long)
Condition
of
the
teeth
Relationship
of
maxillary
incisors
to
mandibular
incisors
(concern-
ing
if
there
is
prominent
overbite)
Ability
to
advance
mandibular
incisors
in
front
of
maxillary
incisors
(concerning
if
unable
to
do
this)
Interincisor
or
intergum
(if
edentulous)
distance
(concerning
if
<
3
cm)
Visibility
of
the
uvula
(concerning
if
Mallampati
class
is
3
or
more)
Shape
of
uvula
(concerning
if
highly
arched
or
very
narrow)
Presence
of
heavy
facial
hair
Compliance
of
the
mandibular
space
(concerning
if
it
is
stiff,
indu-
rated,
occupied
by
mass,
or
nonresilient)
Thyromental
distance
(concerning
if
<
6
cm)
Length
of
the
neck
Thickness
or
circumference
of
the
neck
Range
of
motion
of
the
head
and
neck
(concerning
if
unable
to
touch
tip
of
chin
to
chest
or
cannot
extend
neck)
BOX
31.1
Components
of
the
Airway
Examination
From:
Apfelbaum
JL,
Hagberg
CA,
Caplan
RA,
et
al.
Practice
guidelines
for
management
of
the
difficult
airway:
an
updated
report
by
the
American
Society
of
Anesthesiologists
Task
Force
on
Management
of
the
Difficult
Airway.
Anesthesiology.
2013;118:251–270.](https://image.slidesharecdn.com/millercharts-220915004659-e44e6ffc/85/Miller-charts-pdf-267-320.jpg)












