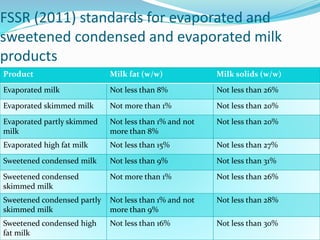
Condensed milk is made by evaporating water from milk, with or without added sugar. Evaporated milk is made similarly but is then sterilized. Condensed milk has a higher concentration of milk solids than evaporated milk. The manufacturing process involves cooling, filtering, standardizing, heating, adding sugar for condensed milk, further concentrating, then packaging. Physico-chemical changes during processing include increased density, decreased pH and increased viscosity. Over time, sweetened condensed milk may experience age thickening and gelation due to higher concentration and added sugar.