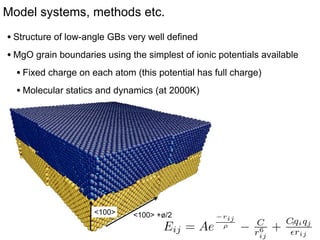
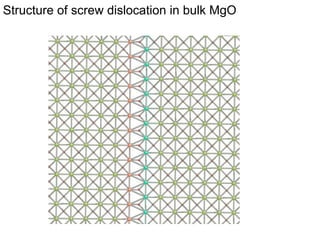
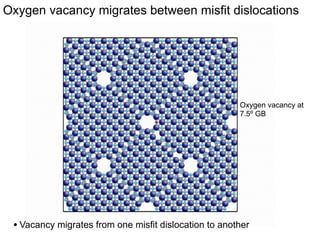
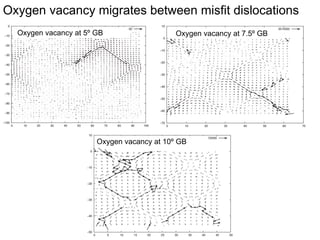
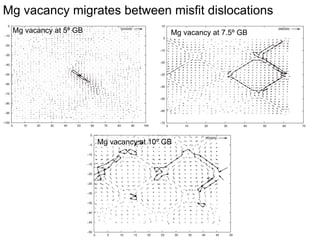
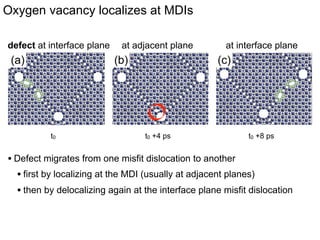
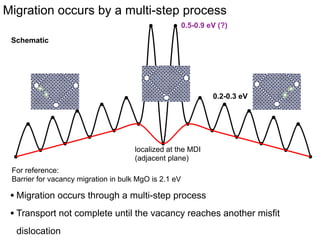
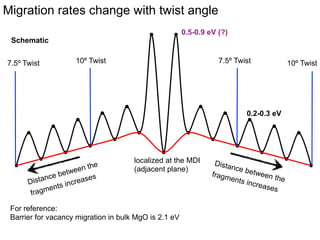
1. The authors studied the structure and transport of oxygen vacancies in MgO grain boundaries using molecular statics and dynamics simulations with a simple ionic potential model.
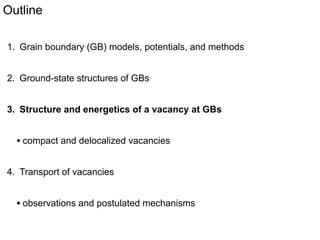
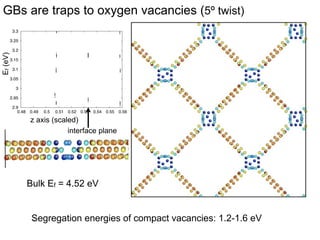
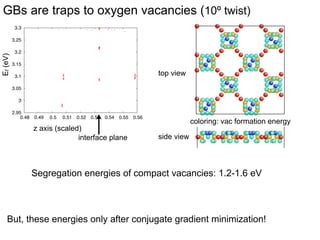
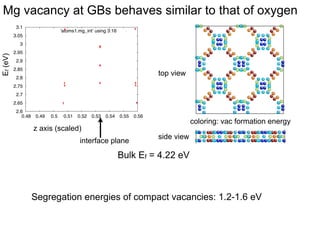
2. They found that grain boundaries act as traps for oxygen vacancies, with segregation energies of compact vacancies in the range of 1.2-1.6 eV.
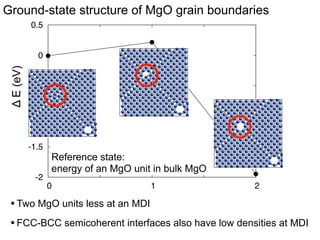
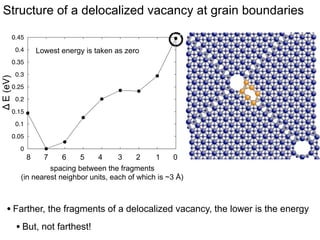
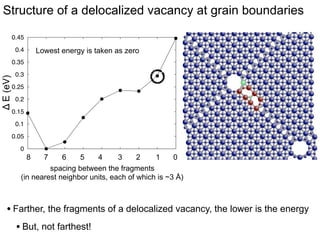
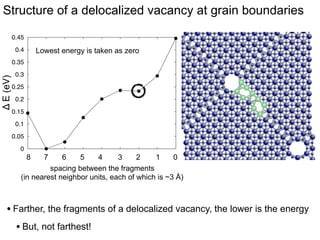
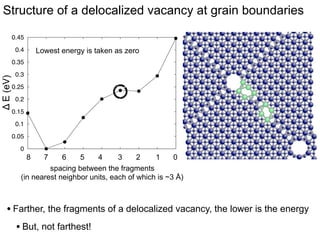
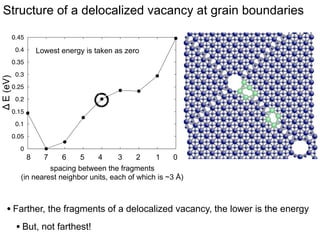
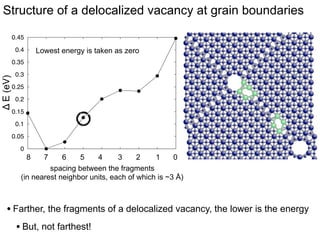
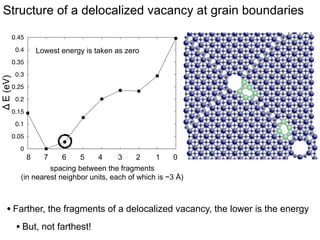
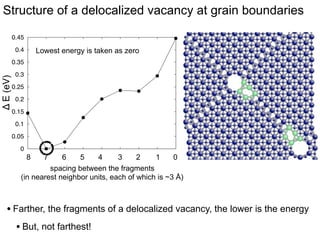
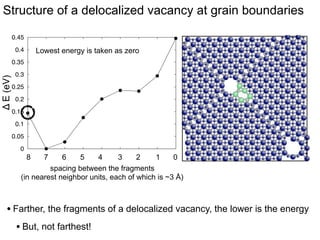
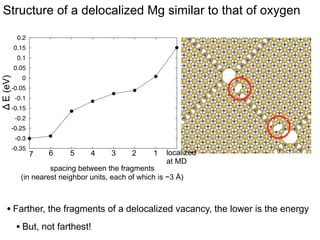
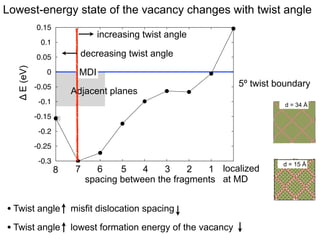
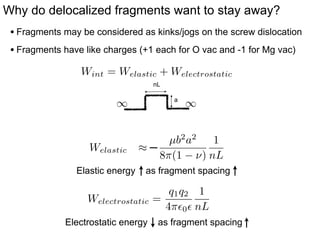
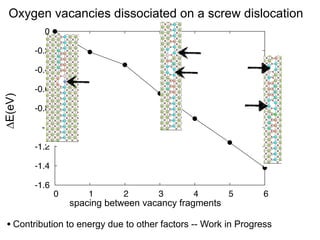
3. The vacancies can also exist in a delocalized state, where the vacancy fragments are separated across multiple atomic planes near the grain boundary. Delocalized configurations were found to have lower energies when the fragments were separated by an intermediate distance.

![the
yers
The
nm.
ystal
hick
minal
ance
as a
ni.
ductrs at
are
con-
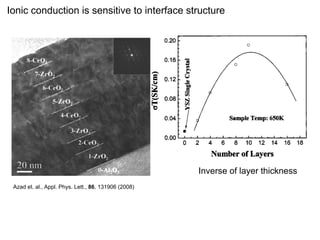
abrupt conductivity decrease when the thickness
ionic conductivity of this material, which imposes results (fig. S1), meaning that the ultrathin layer range or sd
changes from 30 to 62 nm is most likely due to
rather high operational temperatures around 800°C a of YSZ grows rotated by 45° around the c axis obtained fr
degraded search for alternative electrolytes has
(1–4). Theinterface structure when the YSZ layers and strains to match the STO lattice. Because the frequency p
exceed the critical thickness.
not yet been successful in reaching the conduc- bulk lattice constants of STO and YSZ are due to grai
tivity value of 0.01 S/cm desired for room temperature operation (1–4).
Only modest reductions in the operation
temperature of SOFCs (500° to 700°C) can be
anticipated with the recently proposed optimized
electrolytes such as gadolinia-doped ceria and
lanthanum gallates (8–11). On the other hand, the
one to two orders of magnitude increase of the
electrical conductivity reported (12–14) in nanocrystalline samples as compared with single crystals outlines the importance of processing as an
alternative route to increasing conductivity values
toward the desired levels. Because modern thin
film growth techniques allow a precise control of
layer thickness and morphology, they provide a
pathway for the production of solid electrolytes
with optimized properties. Maier et al. found a
substantial increase of the dc ionic conductivity
of superlattices of CaF2 and BaF2 when the Fig. 1. (A) Z-contrast scanning transmission electron microscopy (S
thickness of the individual layers was decreased the [YSZ1nm/STO10nm]9 superlattice (with nine repeats), obtain
J. assigned to a size effect due to microscope. 676 (2008)
down to 16 nm, Garcia-Barriocanal et. al., Science, 321,A yellow arrow marks the position of the YSZ layer. (In
the space charge regions being smaller than the in the VG Microscopes HB501UX column. In both cases a white arrow
layer thickness (15, 16). Kosacki et al. have spectra showing the O K edge obtained from the STO unit cell at the
reported enhanced conductivity in highly tex- into the STO layer (black squares). (Inset) Ti L2,3 edges for the same
tured thin films of YSZ with thicknesses between are the result of averaging four individual spectra at these position
60 and 15 nm, reaching 0.6 S/cm at 800°C (17).
Because reducing film thickness (and therefore Fig. 2. Real part of the lateral
increasing the fraction of material near the in- electrical conductivity versus fre-
Solid Interfaces can enhance mass transport
www.sciencemag.org on September 17, 2011
scaling,
ductance
the large
res orig-](https://image.slidesharecdn.com/mgomrsv3-130409111956-phpapp01/85/Fall-MRS-2013-MgO-grain-boundaries-structure-and-transport-2-320.jpg)