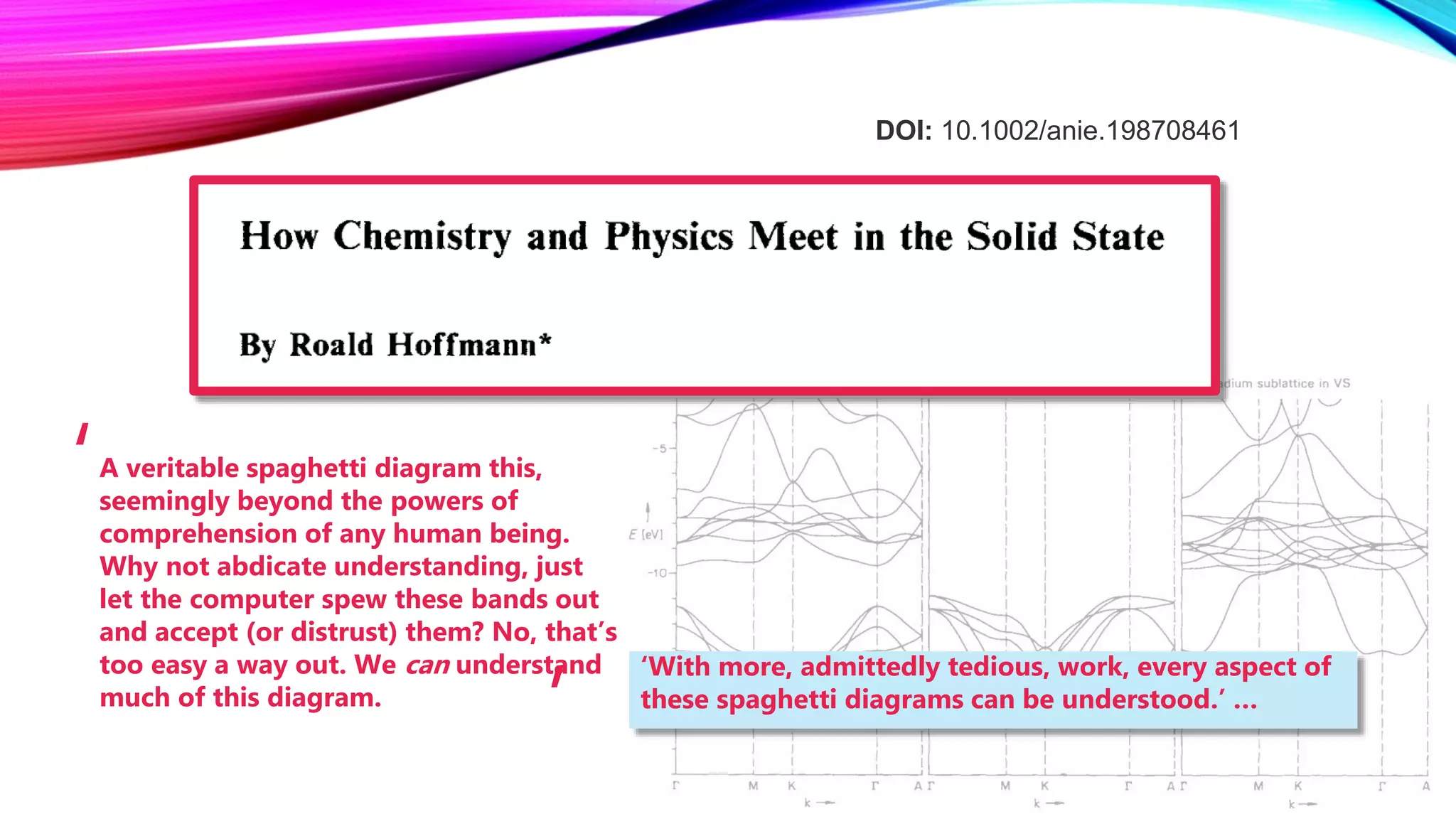
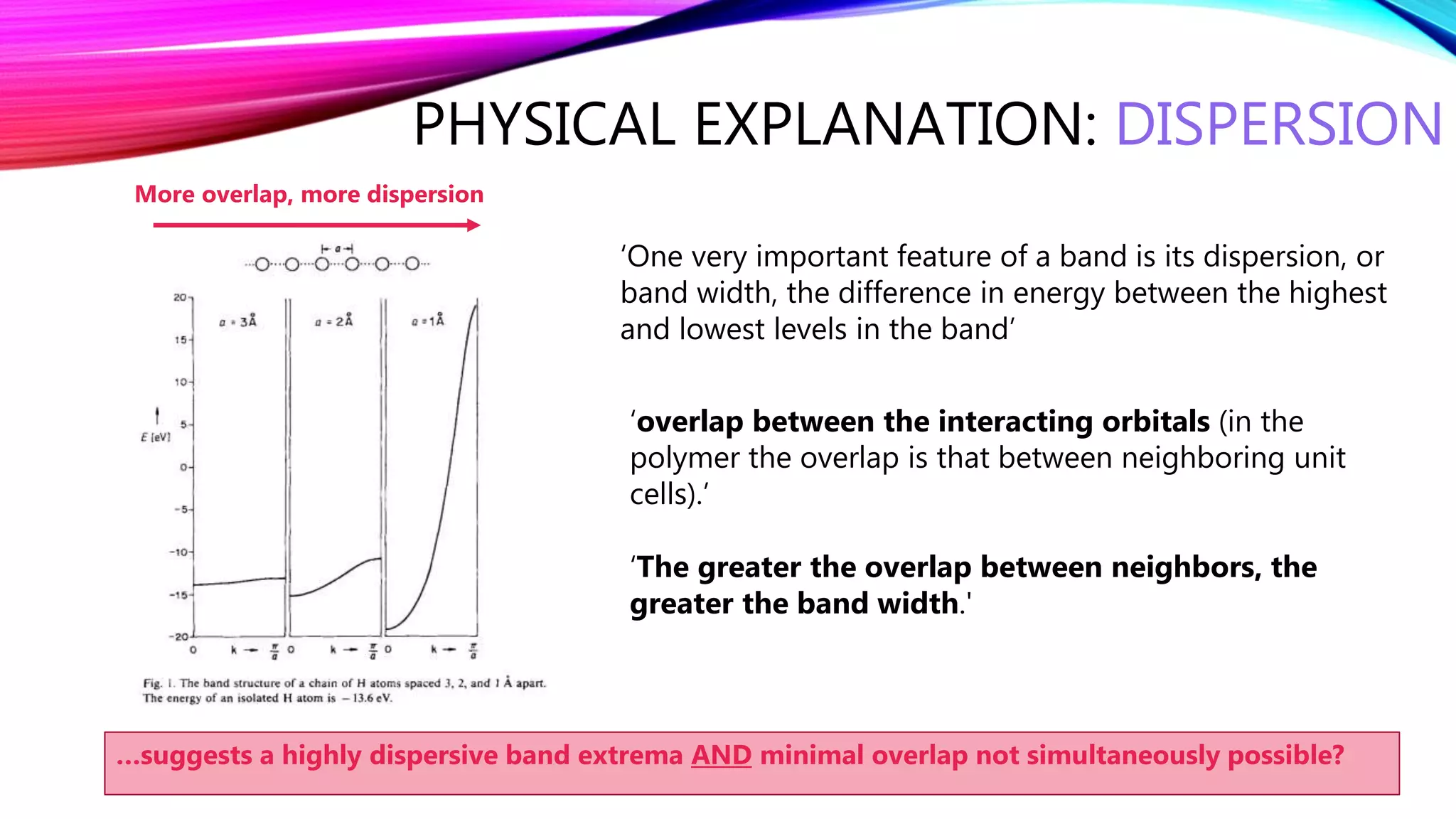
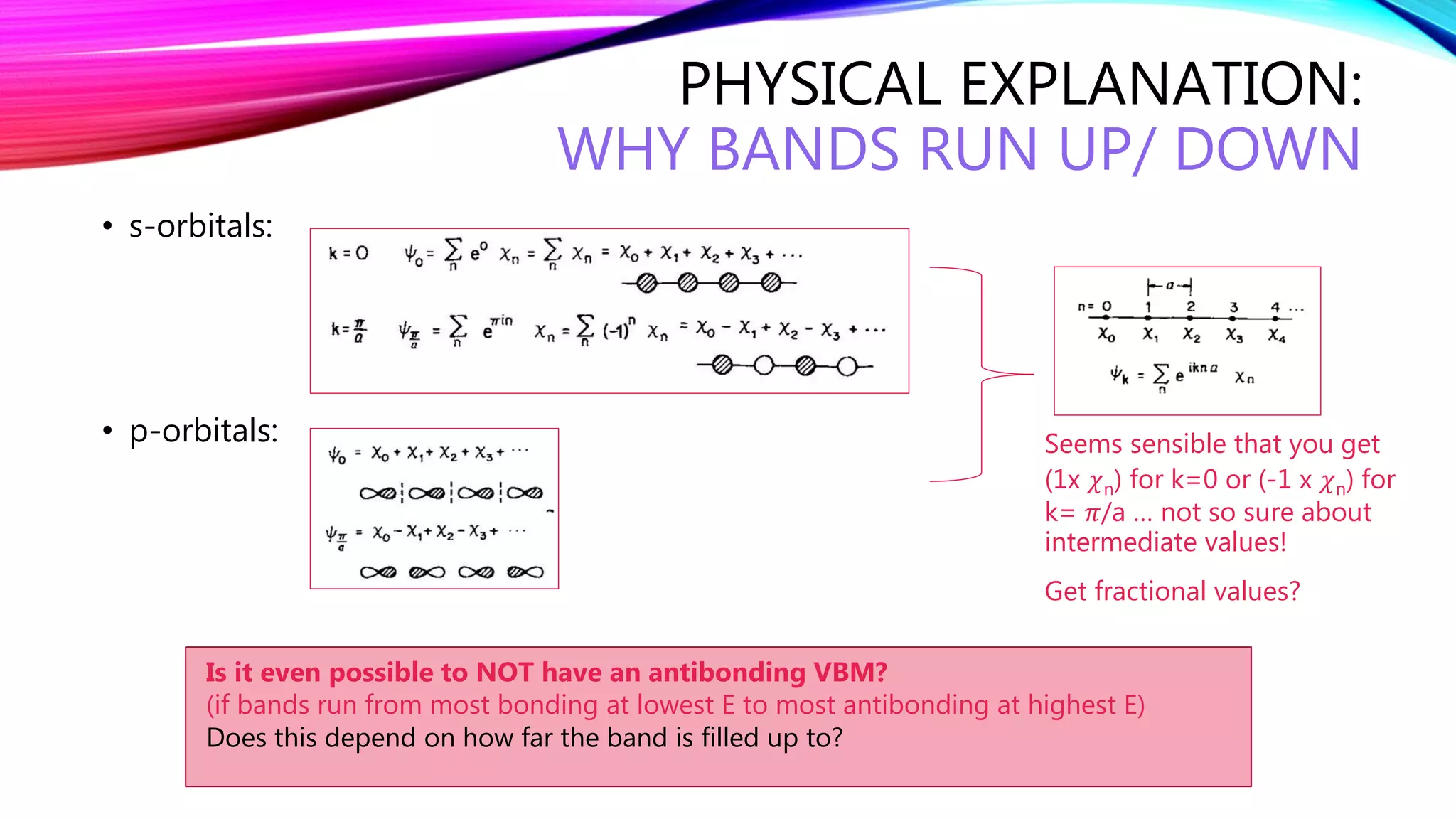
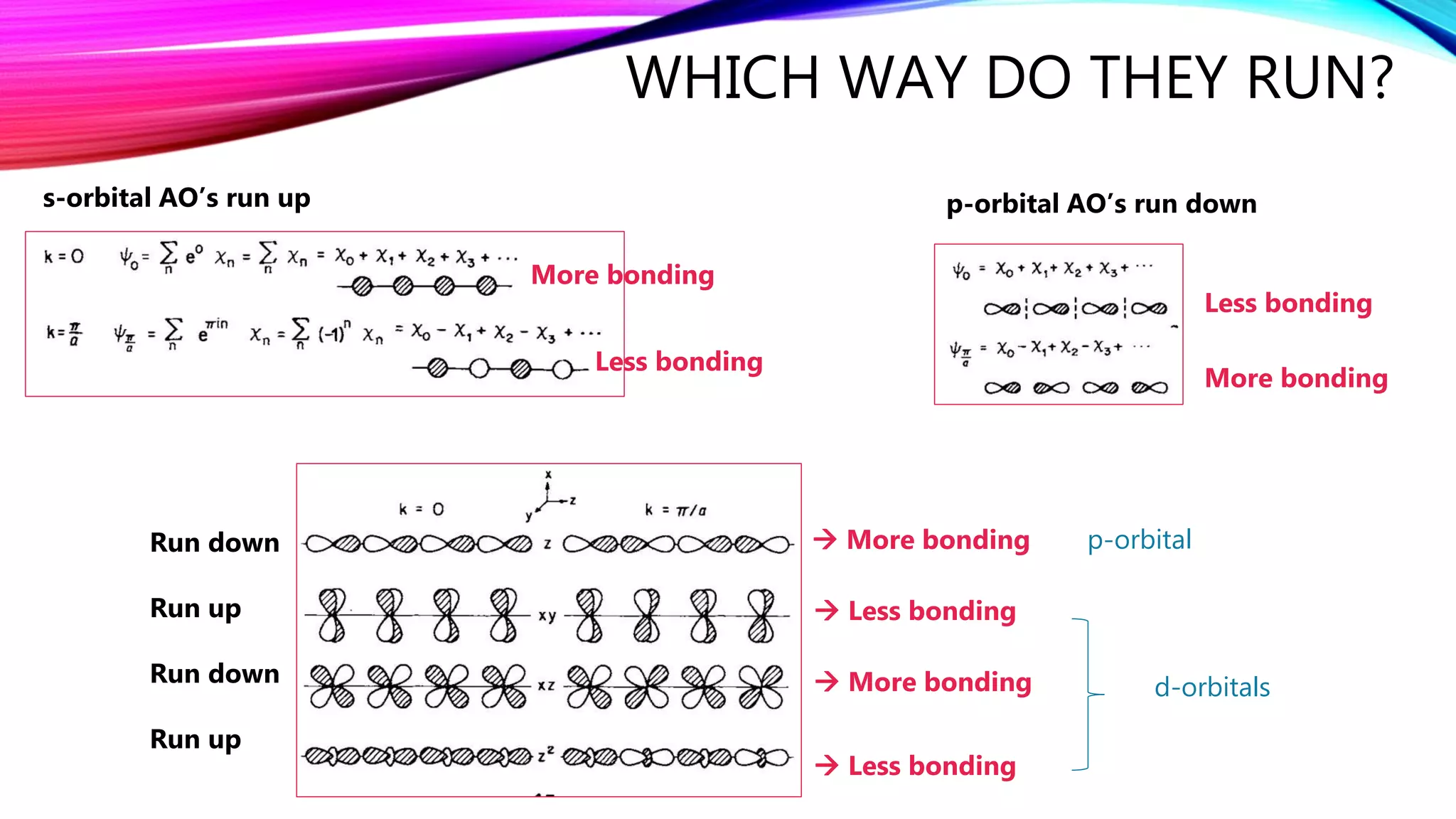
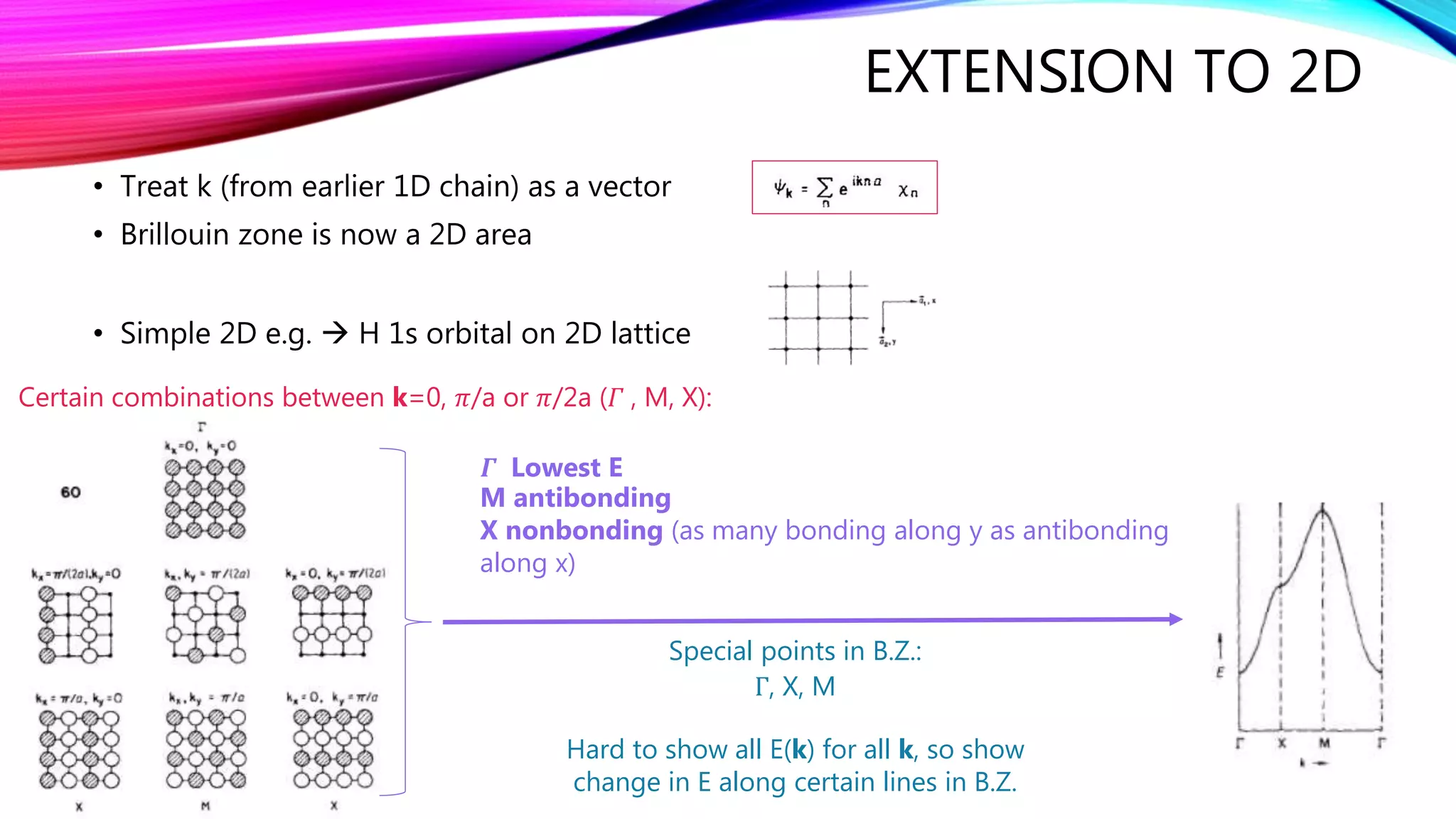
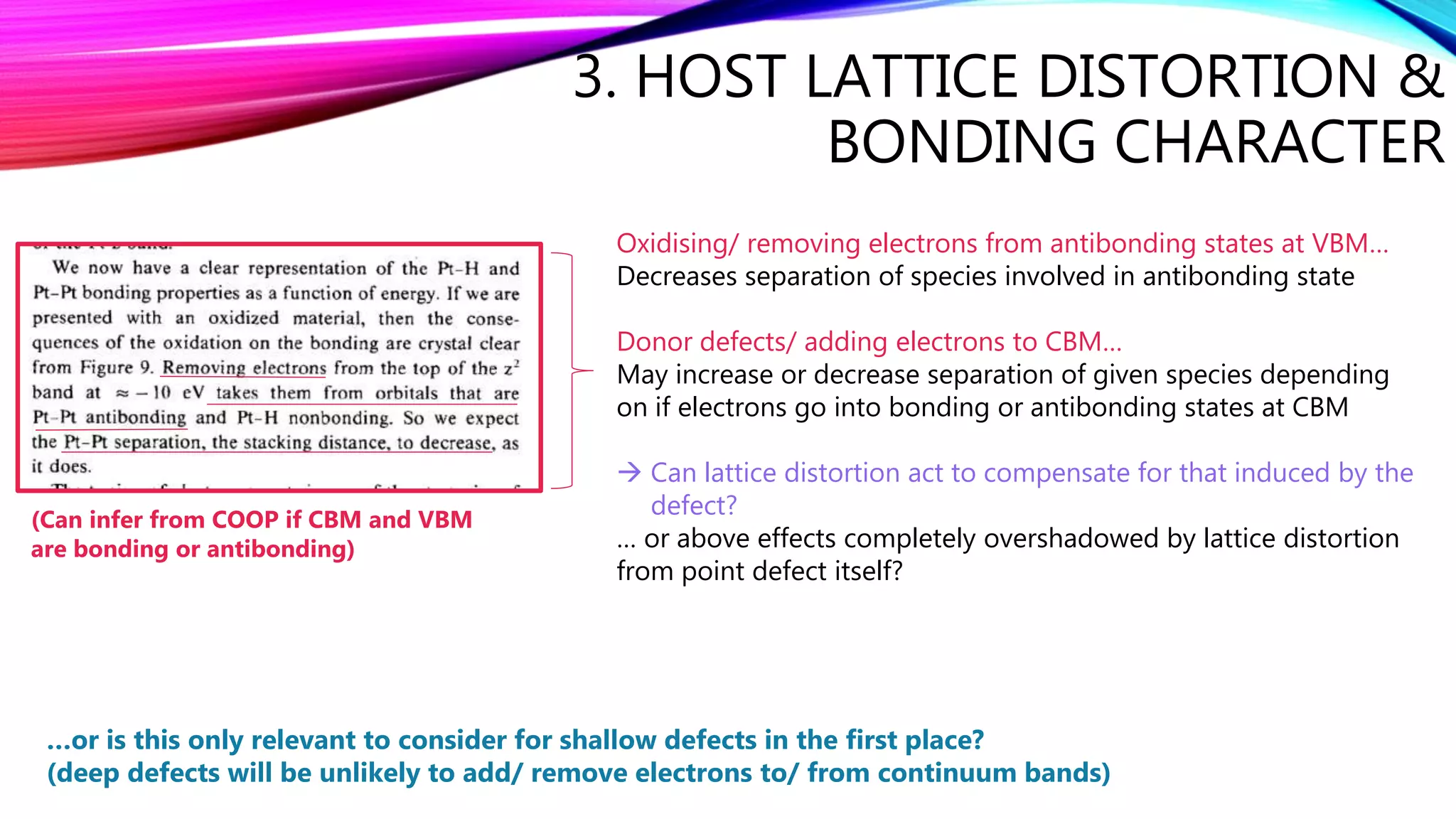
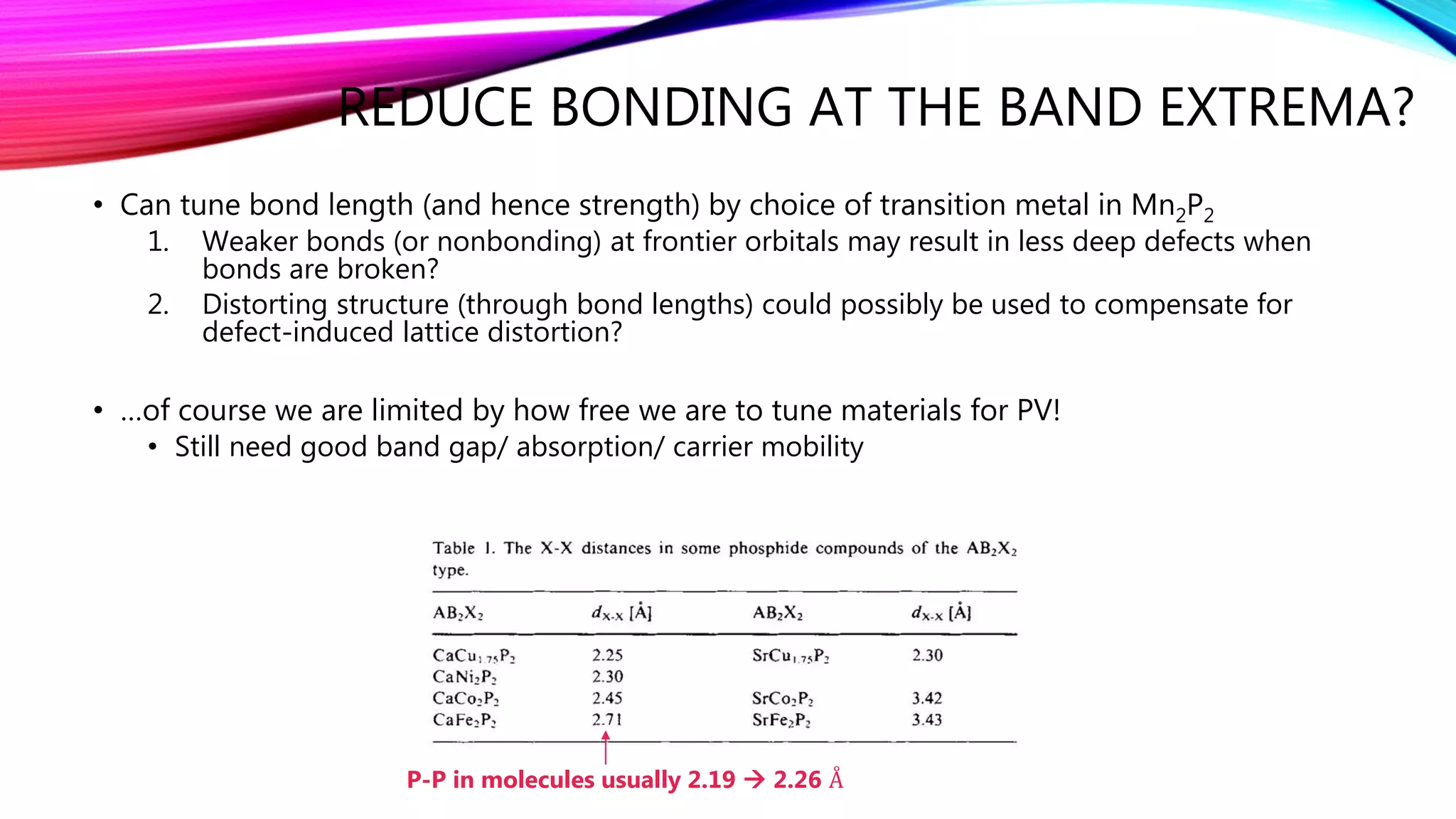
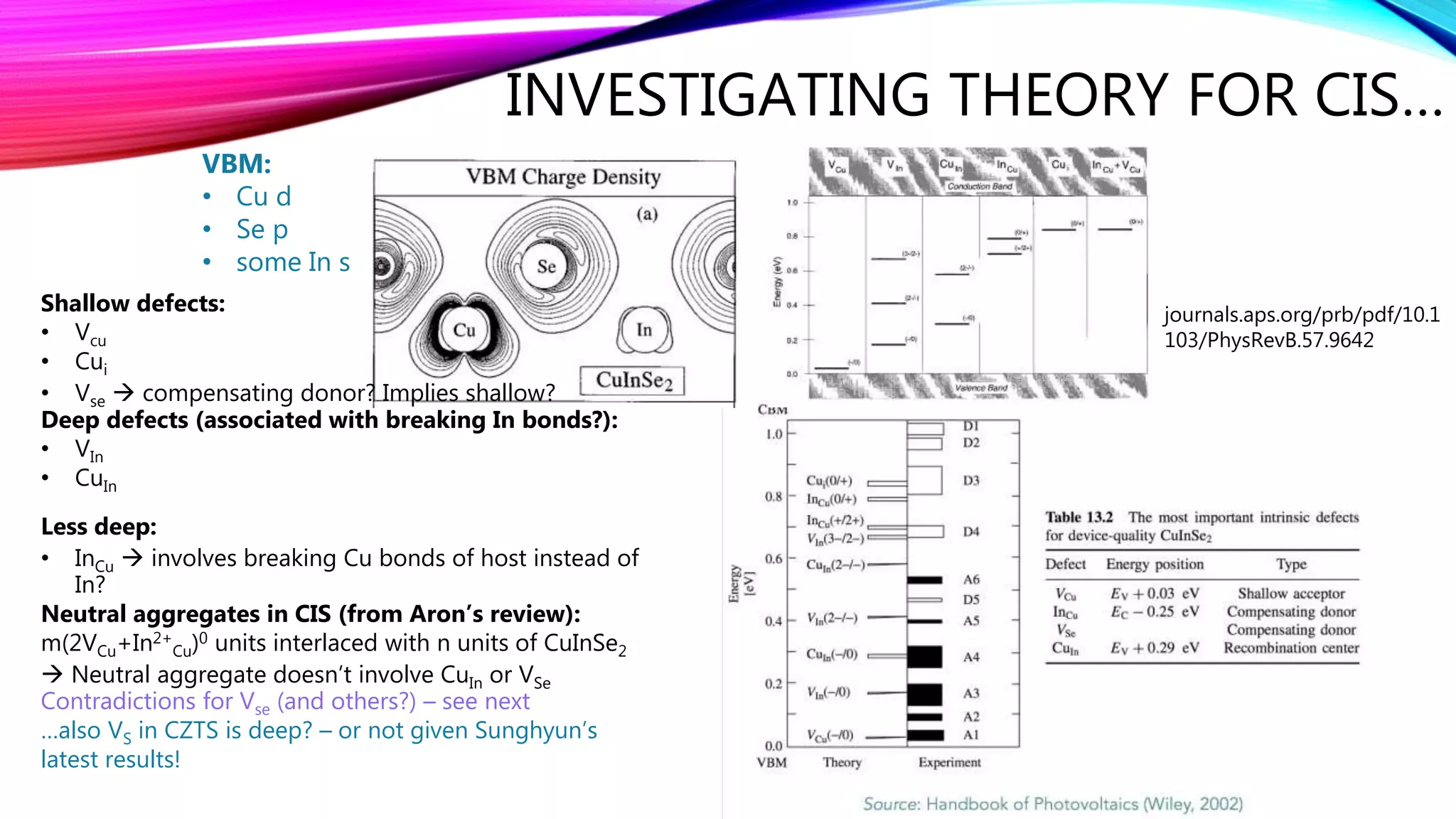
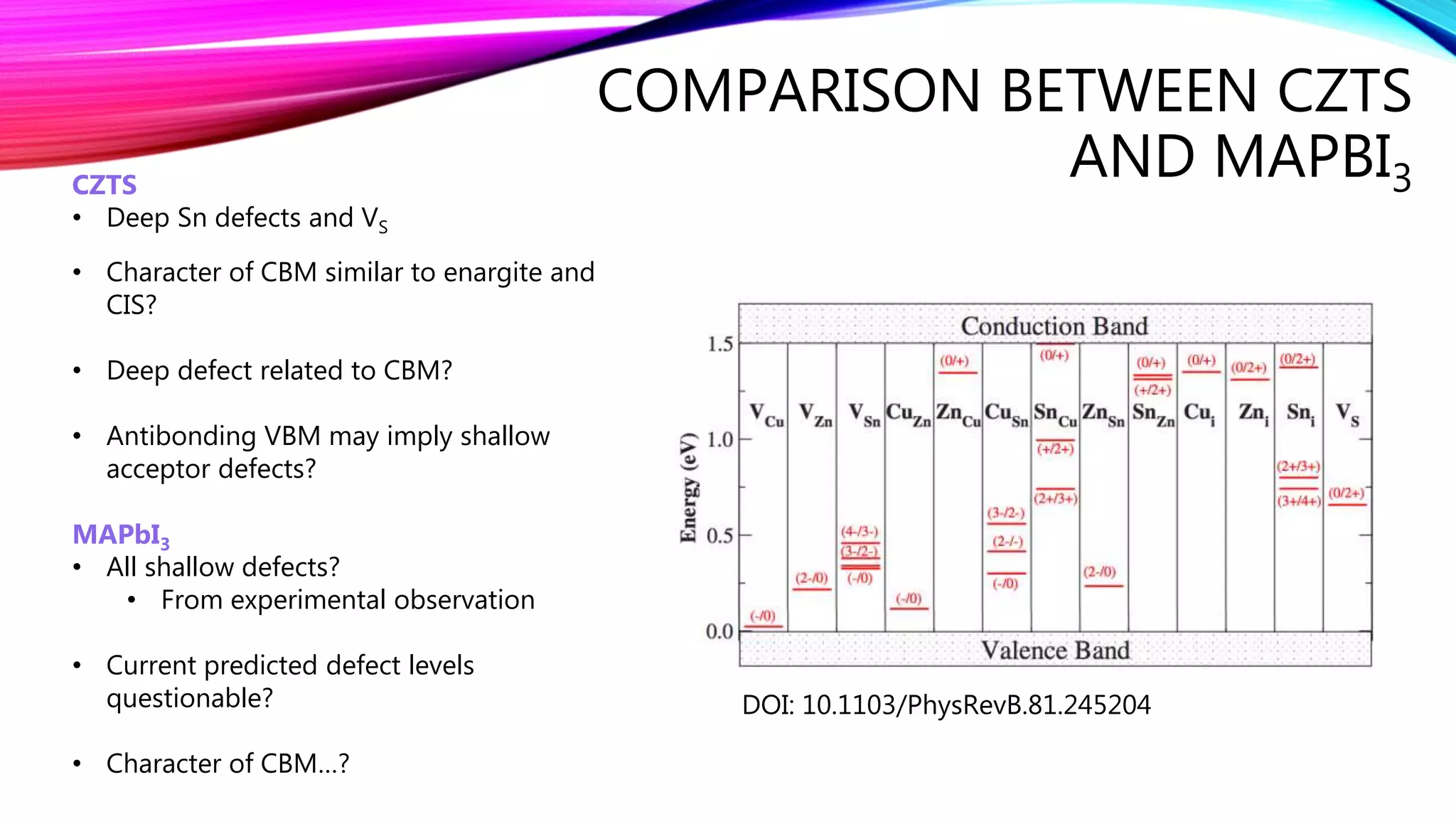
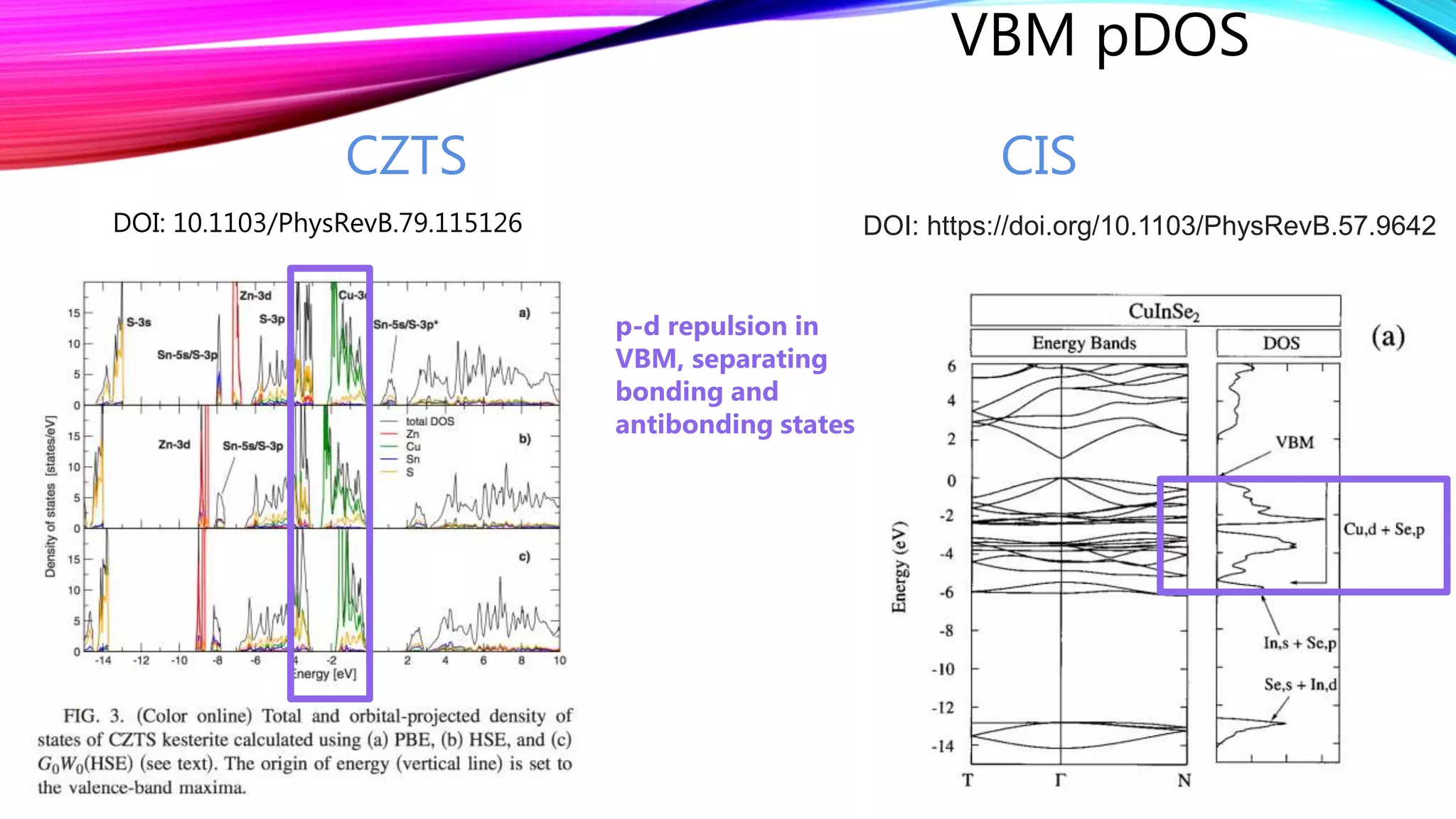
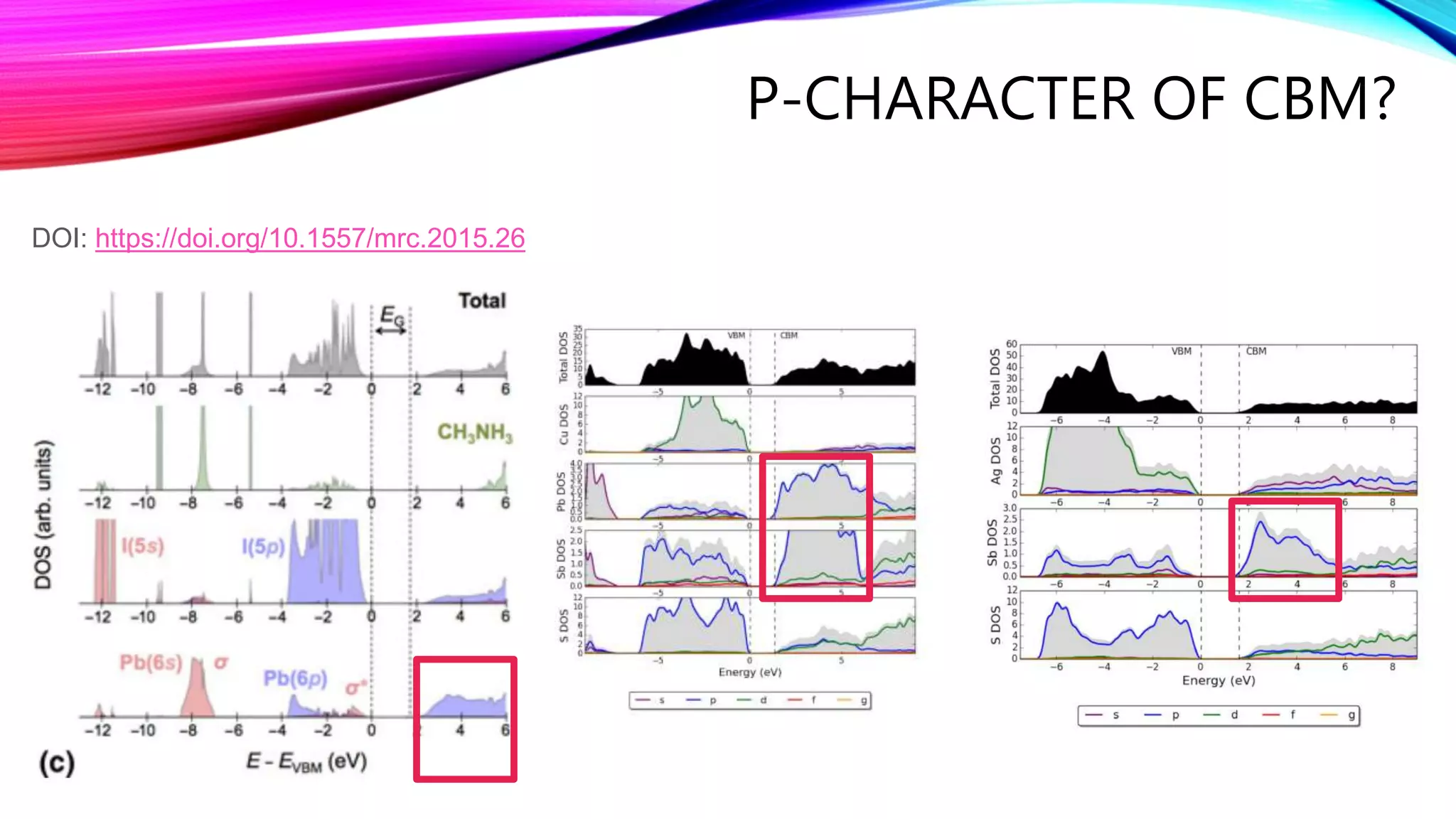
The document discusses predicting band structure without calculation by using Hoffmann's rules. It considers how factors like orbital overlap, dispersion, and structural distortions relate to bonding characteristics in band structures. For defects, it suggests deep levels could arise from many charge transition states, high orbital overlap at band extrema, or large lattice distortions. Extending the ideas to 1D, 2D, and 3D structures, it examines how orbital combinations at high symmetry points relate to bonding vs antibonding character. Structural distortions are noted to maximize bonding by altering densities of states.