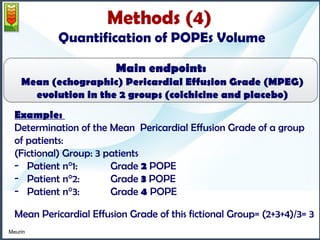
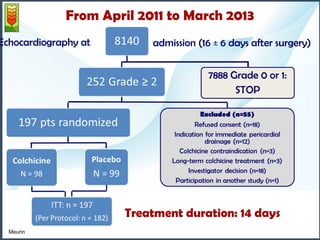
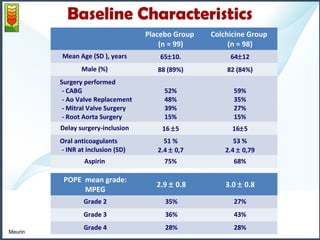
1) The study assessed whether the drug colchicine could reduce the volume of post-operative pericardial effusions (POPEs) in patients at high risk of tamponade. 197 patients were randomly assigned to receive either colchicine or placebo treatment for 14 days.
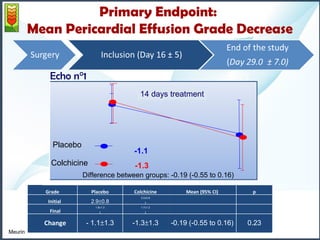
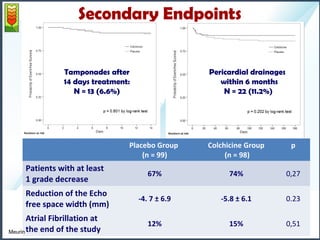
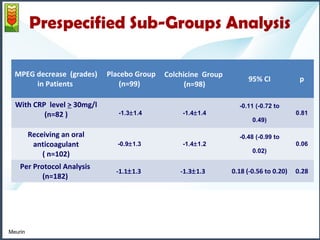
2) The mean pericardial effusion grade (MPEG), as measured by echocardiography, decreased in both groups but the difference between the groups was not statistically significant. Secondary outcomes including tamponade rates were also similar between the groups.
3) The study concludes that colchicine administration appears to be ineffective in reducing POPE volumes and does not provide any benefit over spontaneous resolution.







![Conclusion:
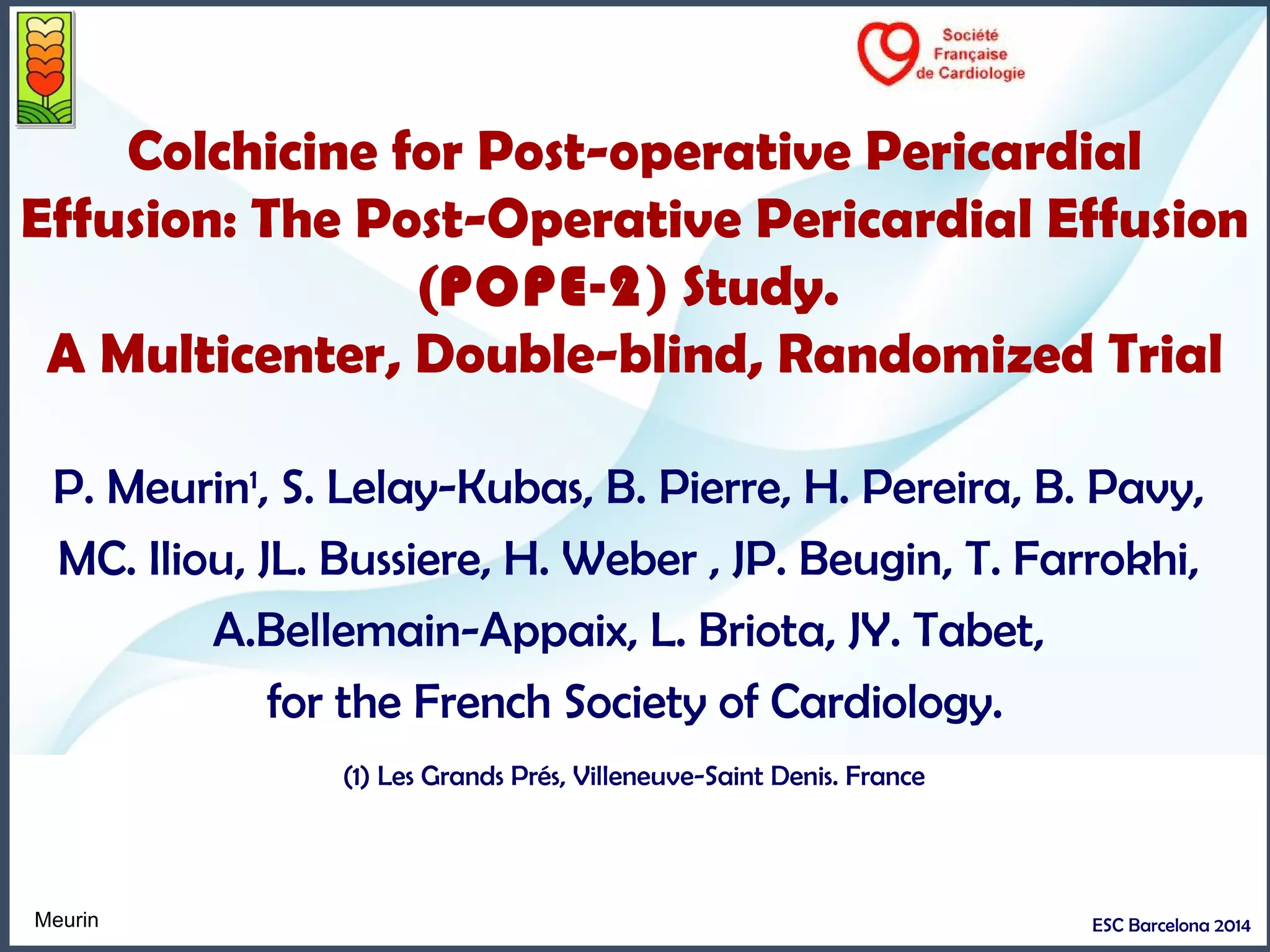
Moderate to large persisting (> 7 days)
post operative pericardial effusion:
What does this study add ?
1- High risk patients: 11,5% reoperation within 6 months:
- 6,6 % tamponades in the 2 following weeks
- Another 5 % will require pericardial drainage within 6 months
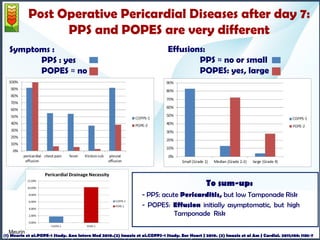
2- Colchicine administration seems to be useless
[PS: NSAID administration seems to be useless (POPE-1 study)]
Meurin](https://image.slidesharecdn.com/meurin-pope2-esc-septembre2014-140921103210-phpapp02/85/Meurin-pope-2-esc-september-2014-20-320.jpg)
