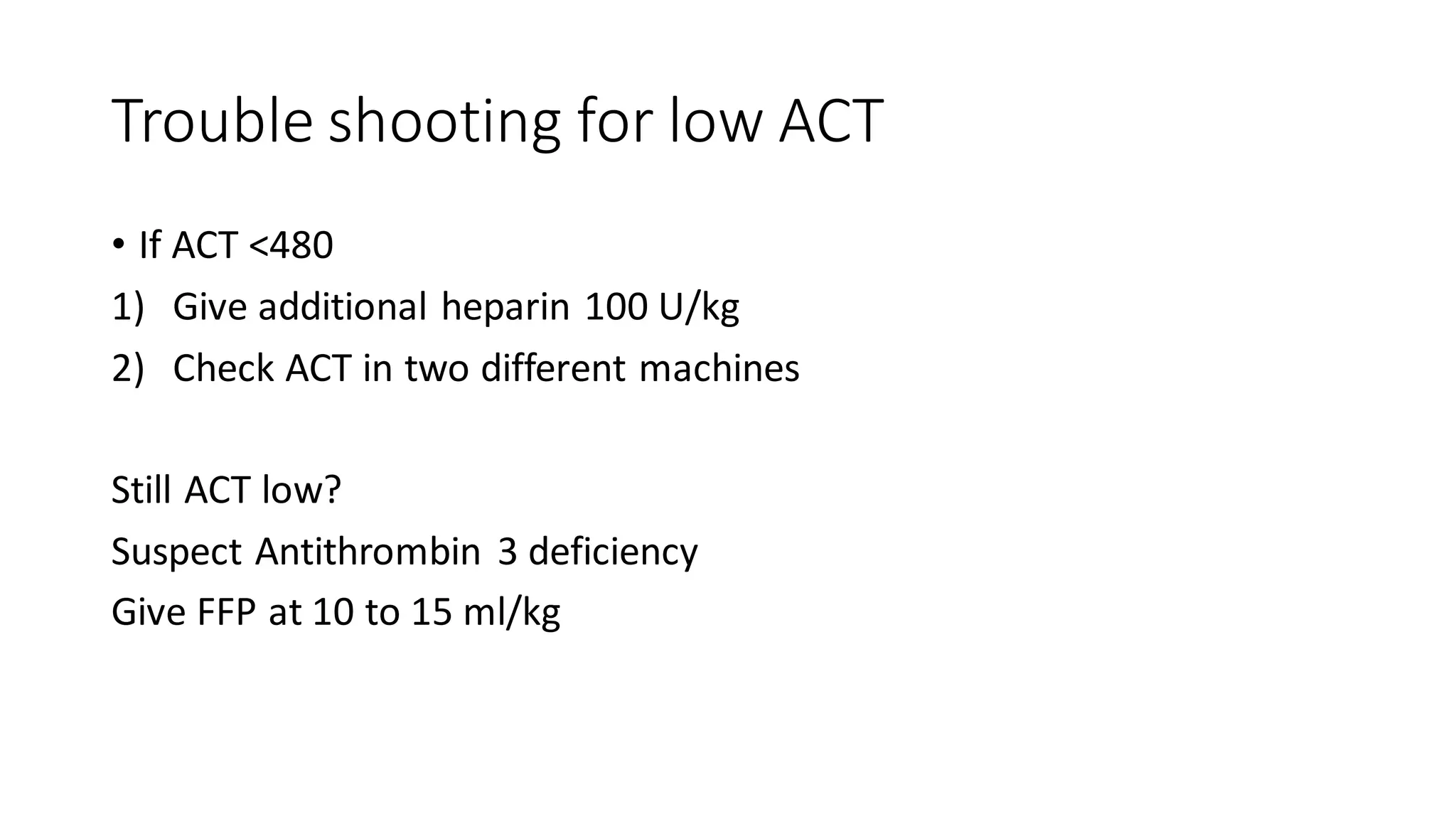
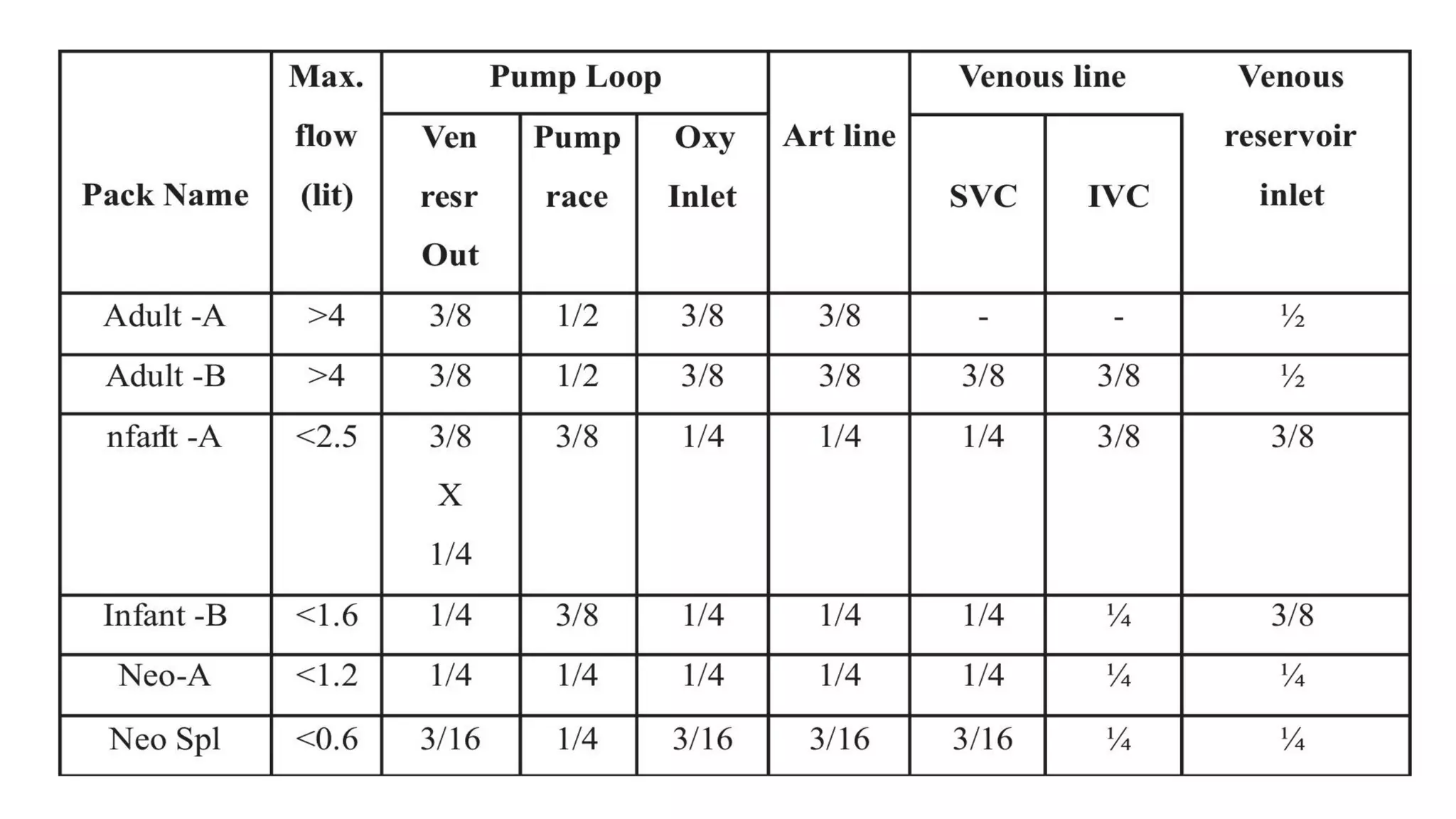
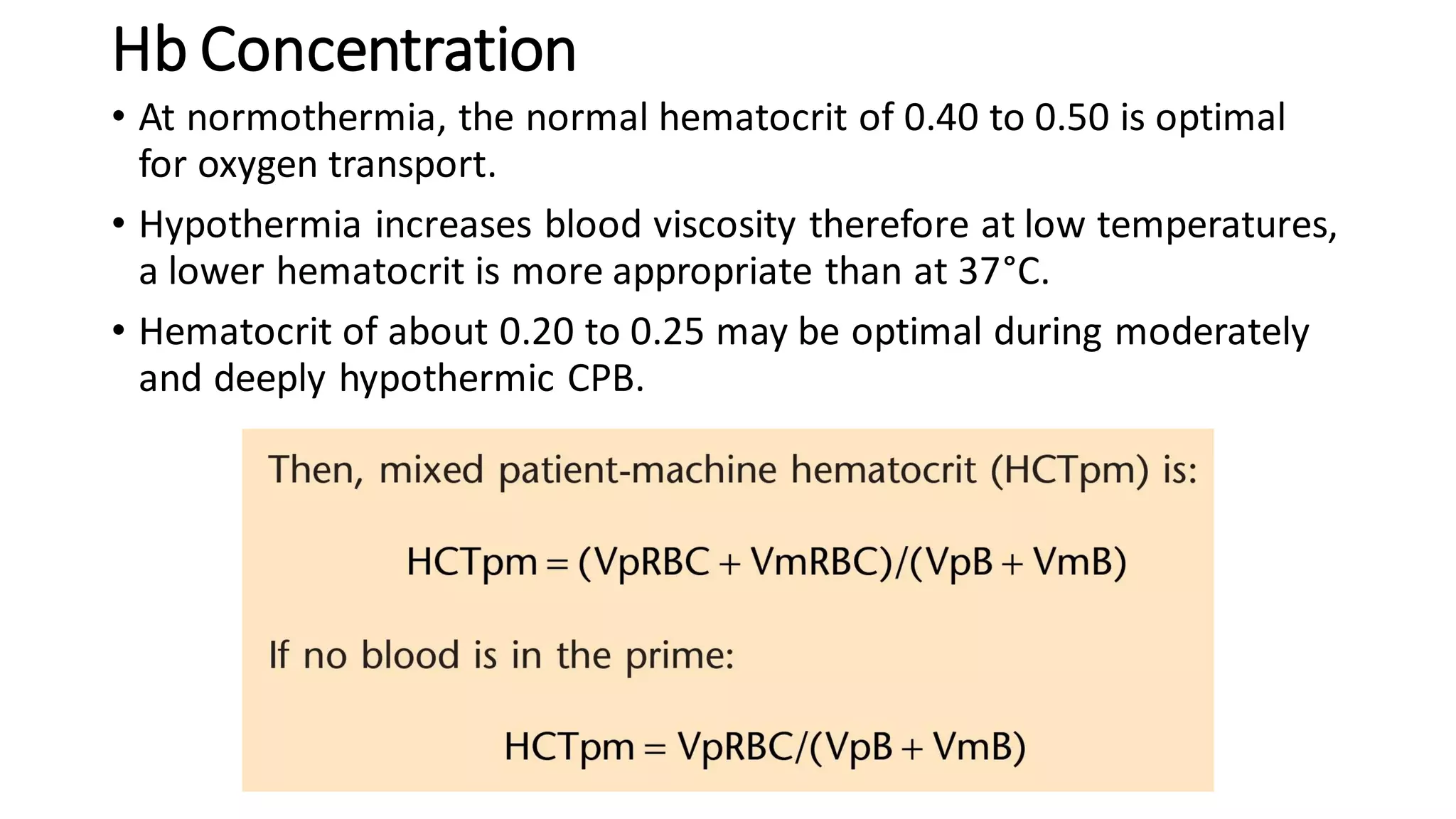
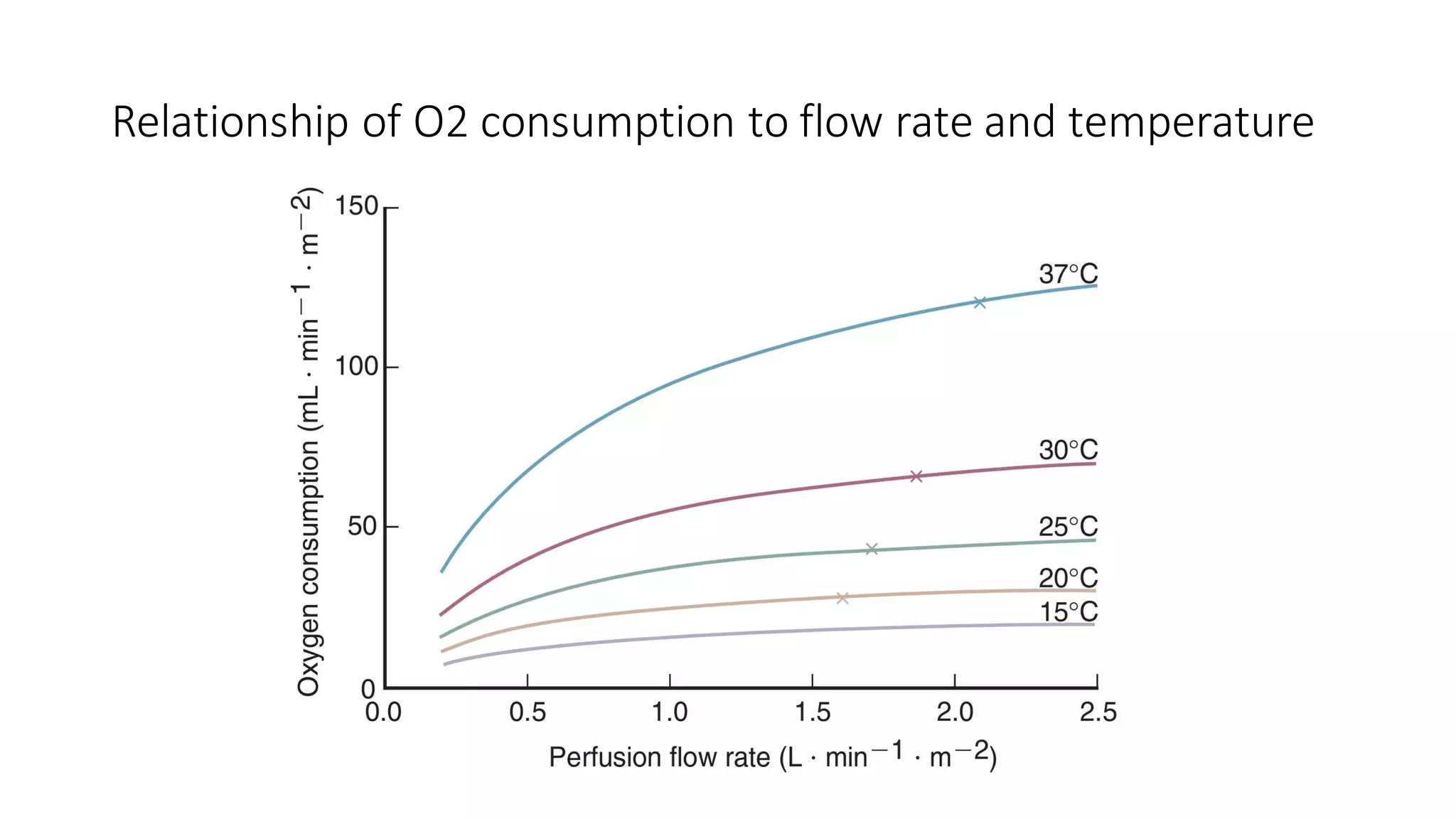
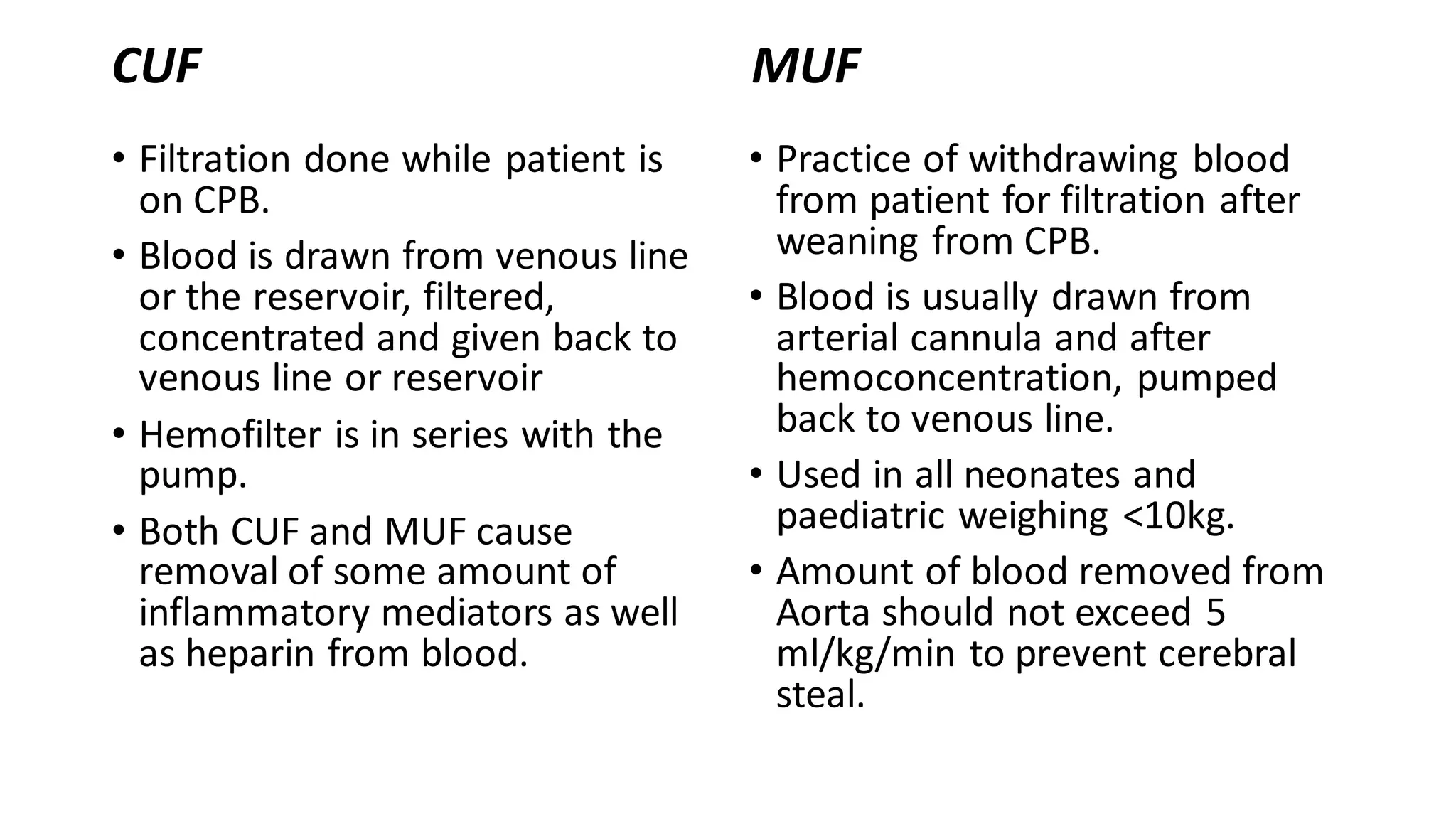
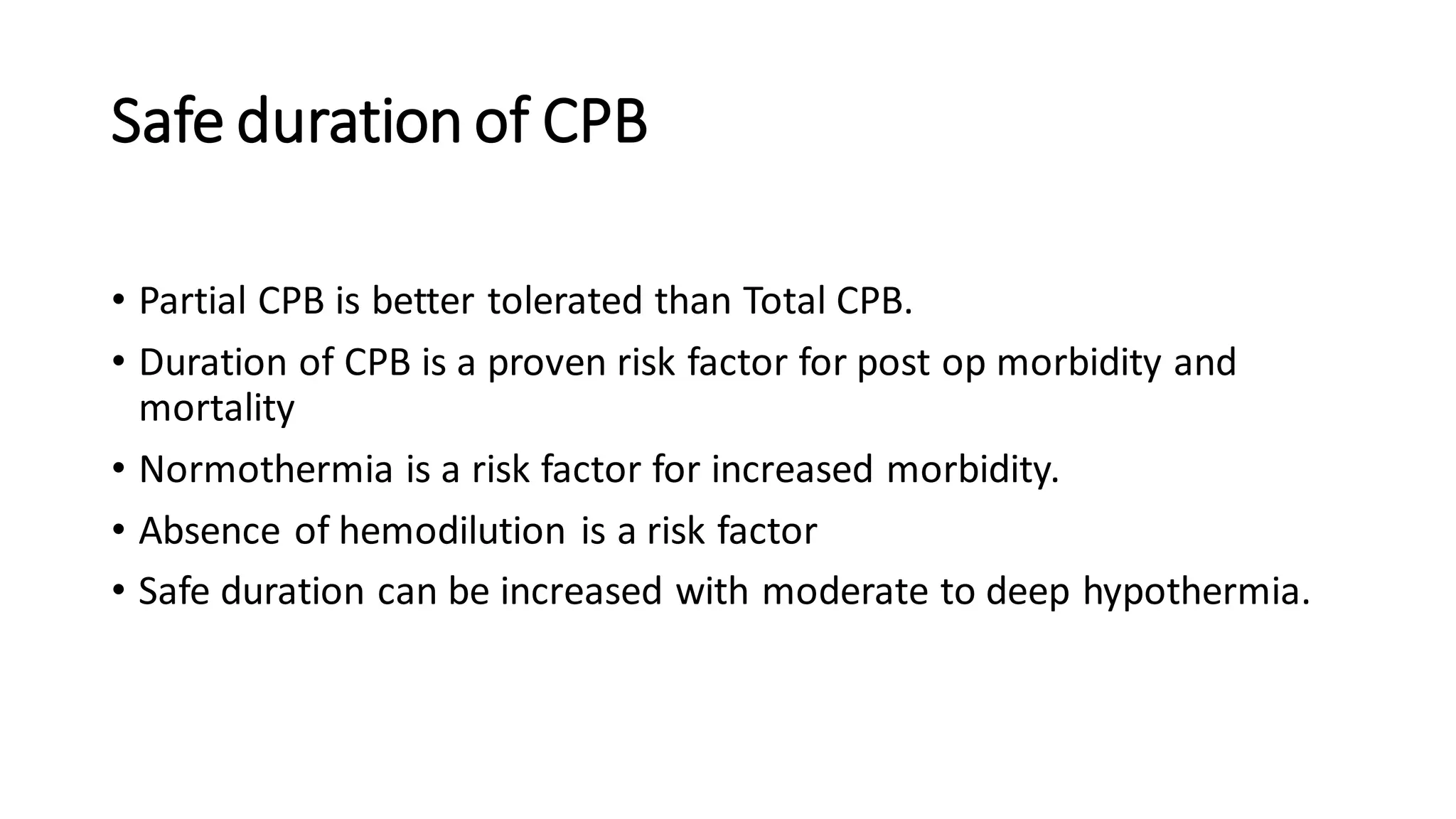
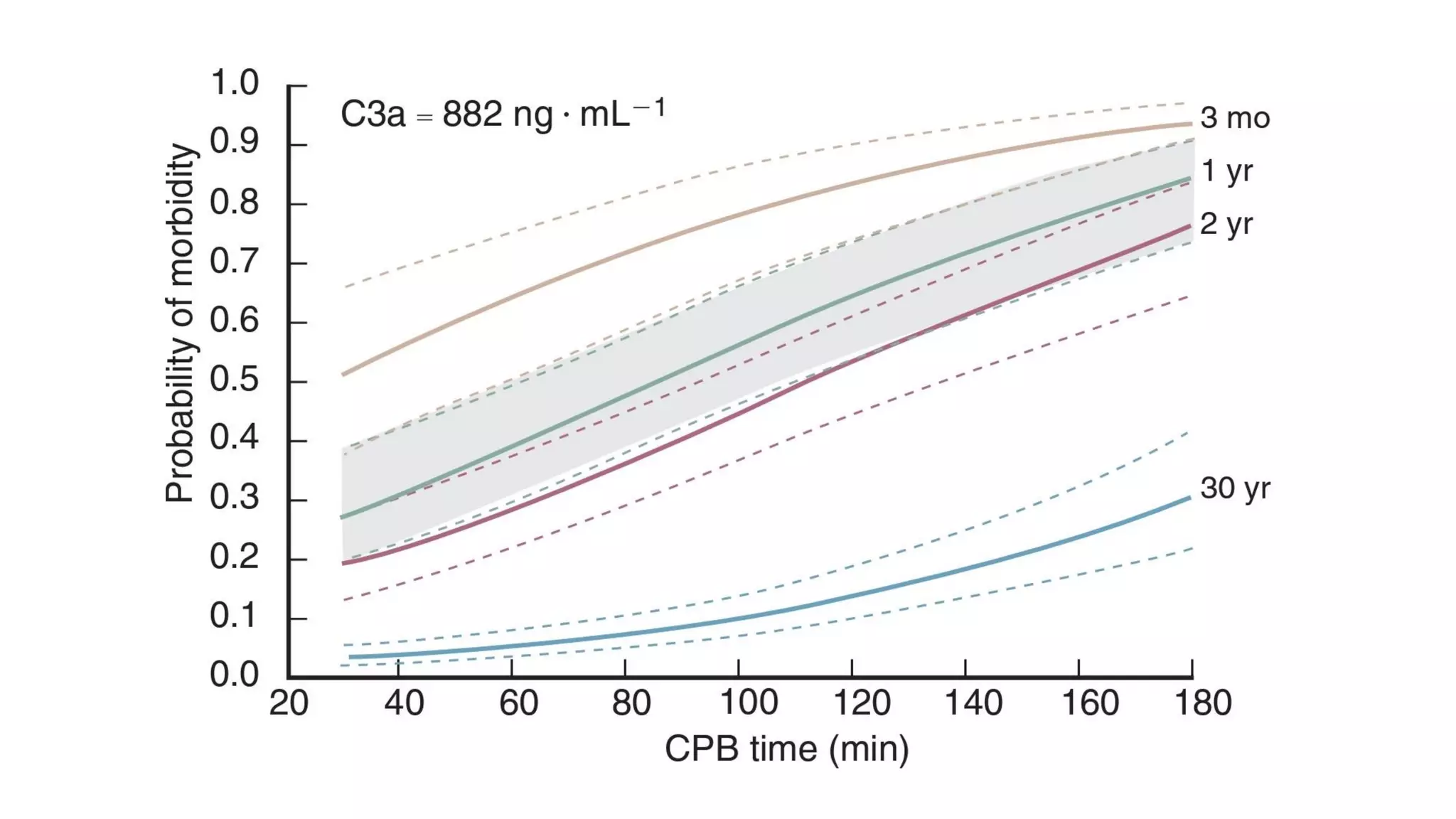
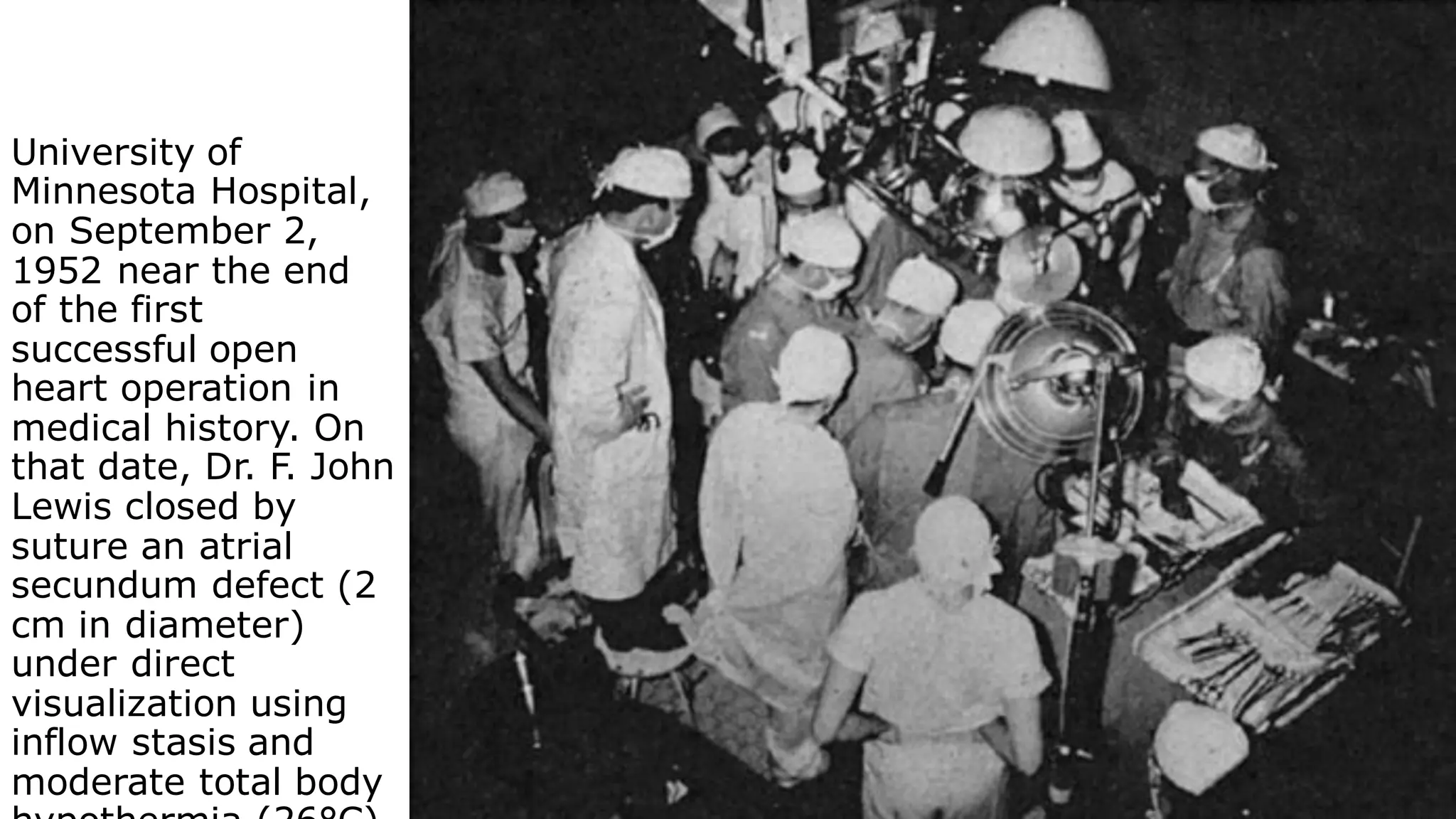
This document provides an overview of cardiopulmonary bypass (CPB), including its history, components of the modern CPB machine, and the CPB procedure. Some key points:
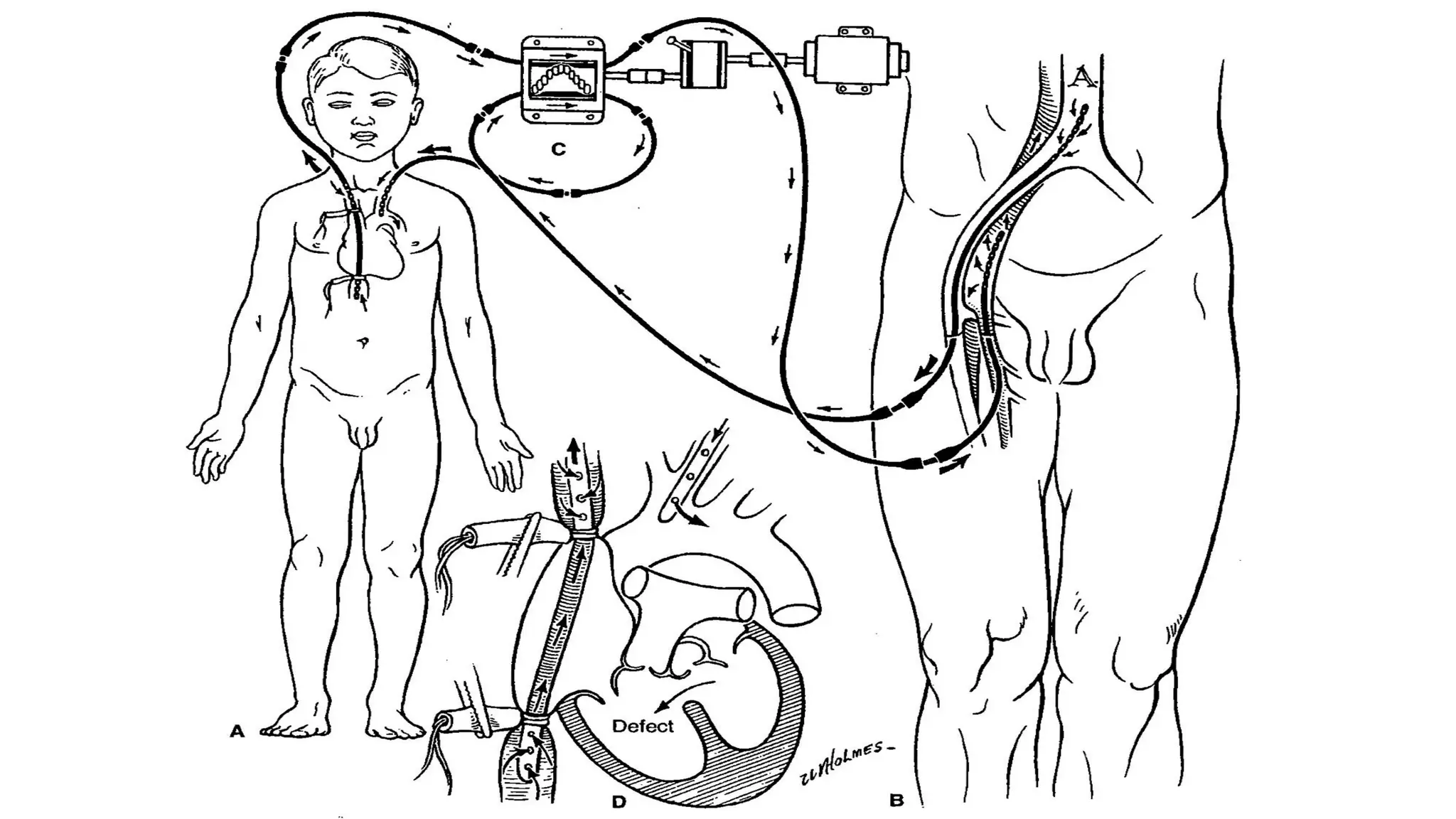
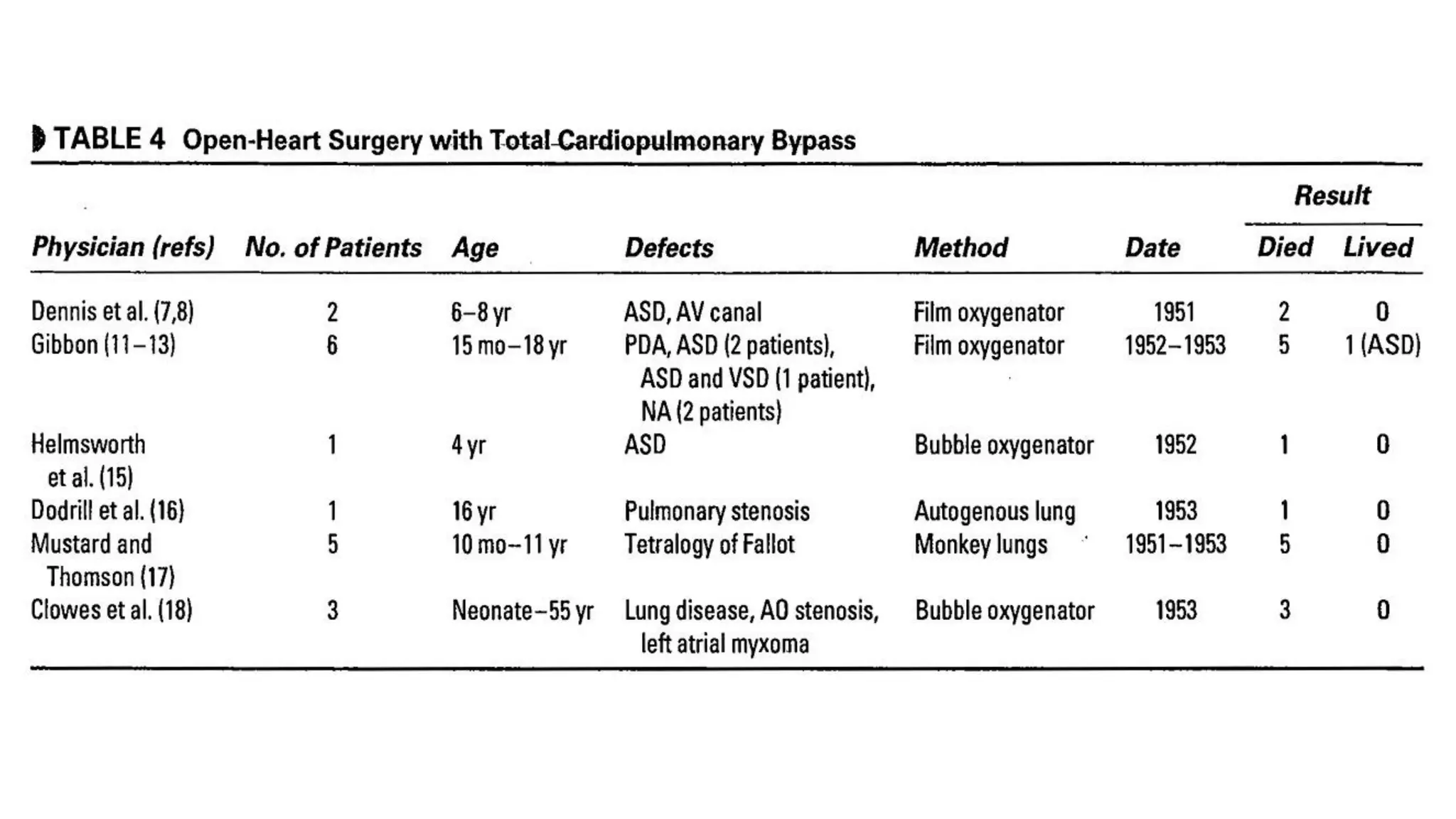
- John Heysham Gibbon Jr. performed the first successful open heart surgery using total cardiopulmonary bypass in 1953.
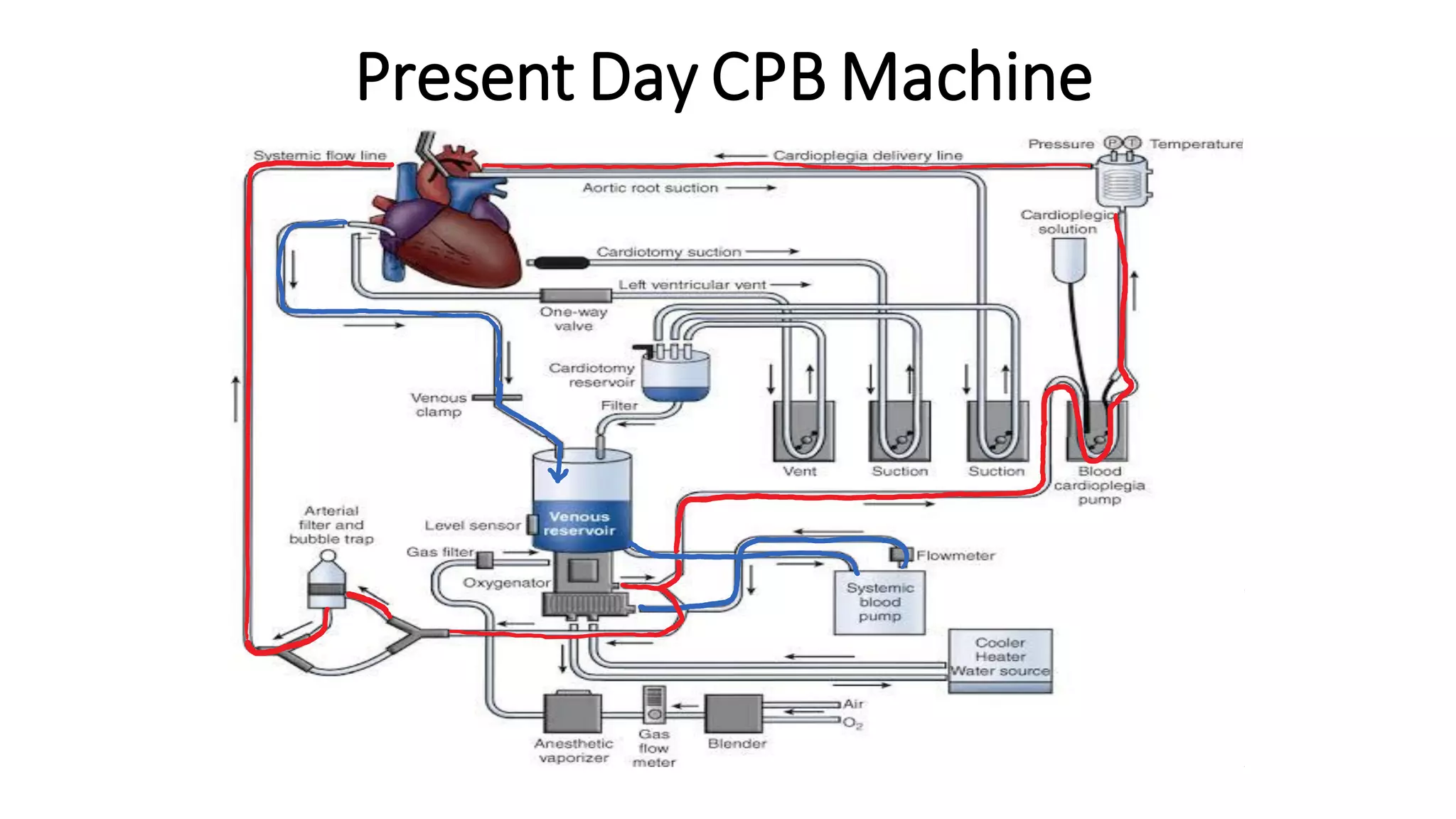
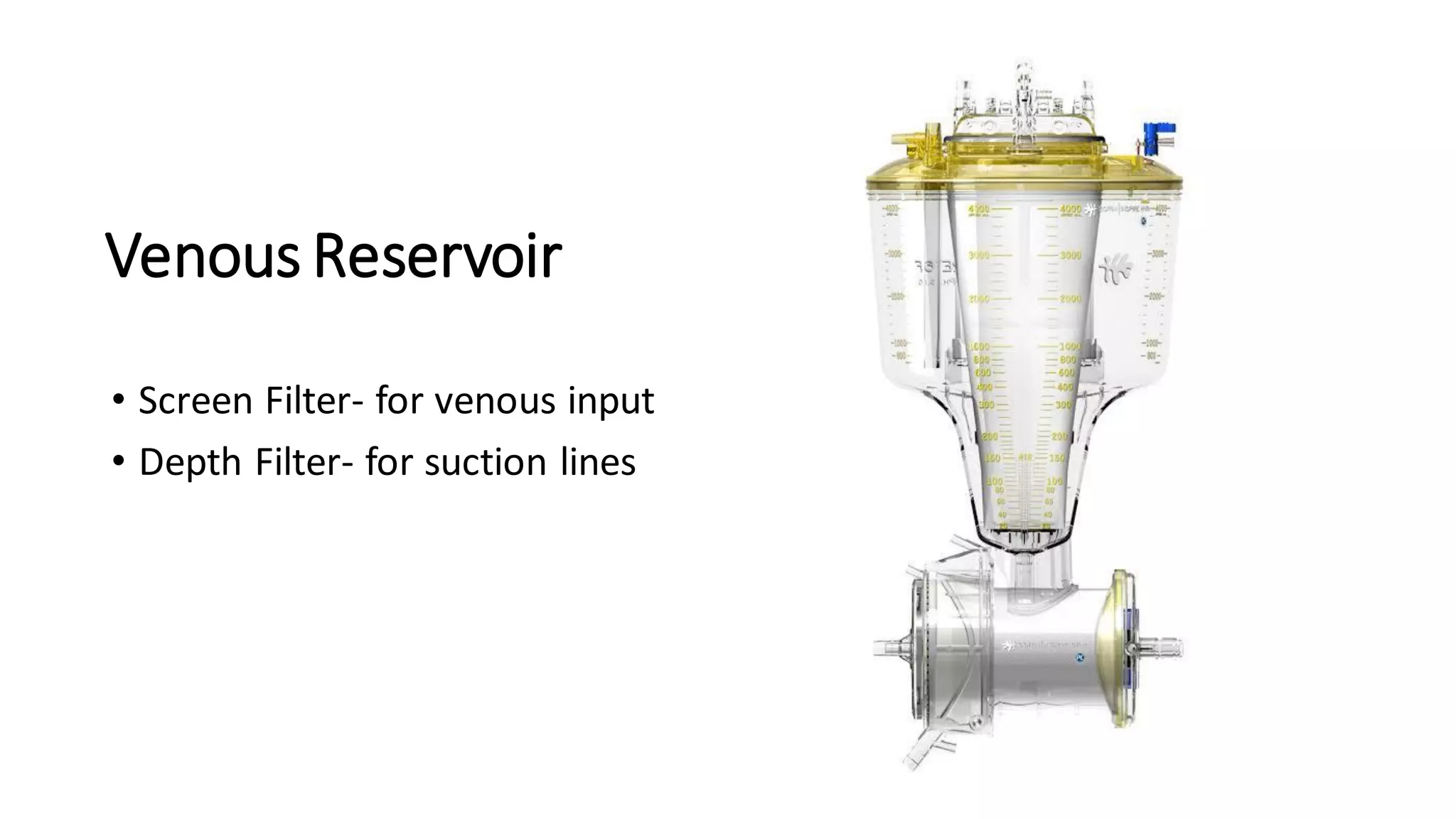
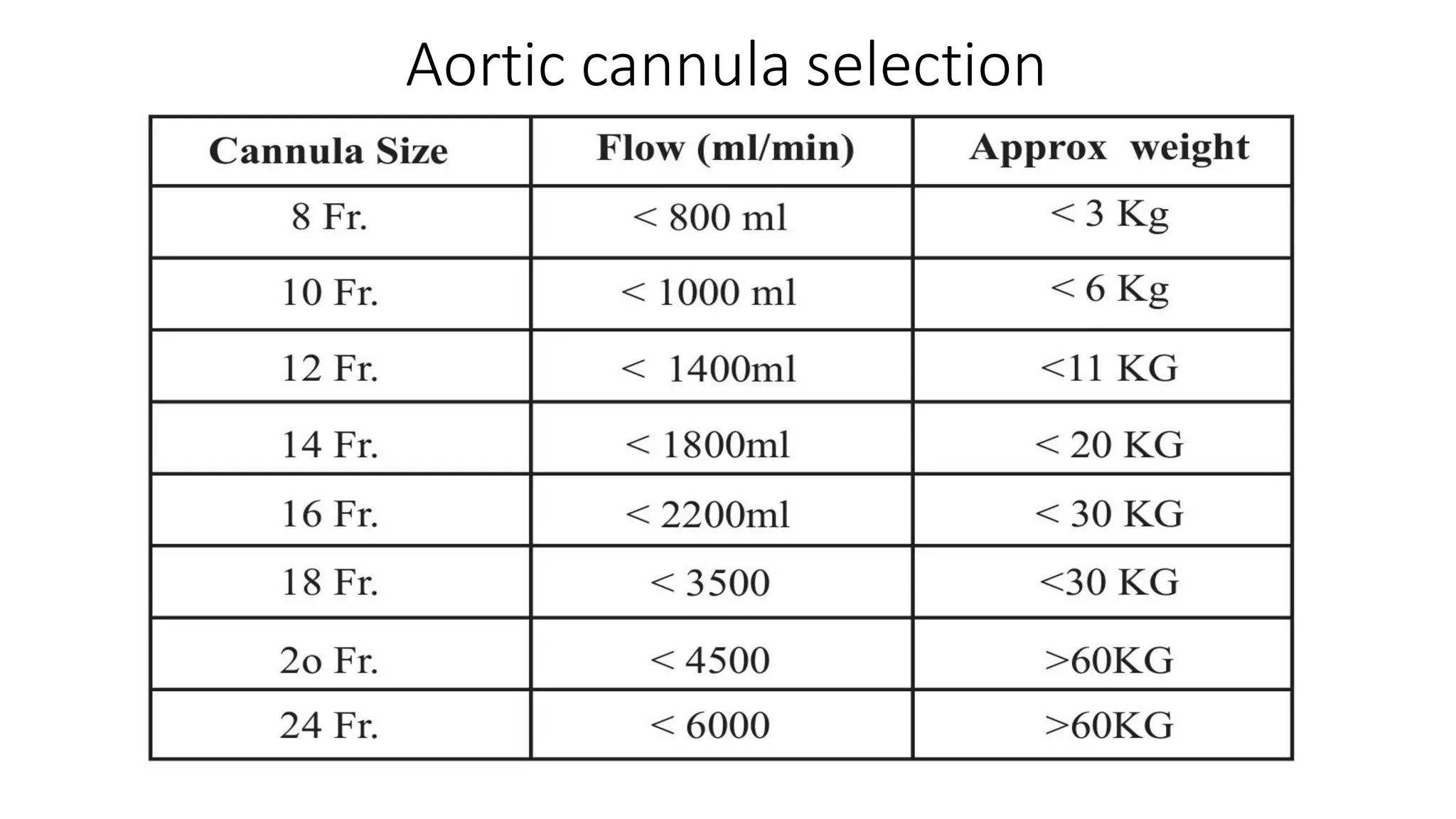
- The main components of the modern CPB machine include the systemic pump, oxygenator, venous reservoir, and arterial filter.
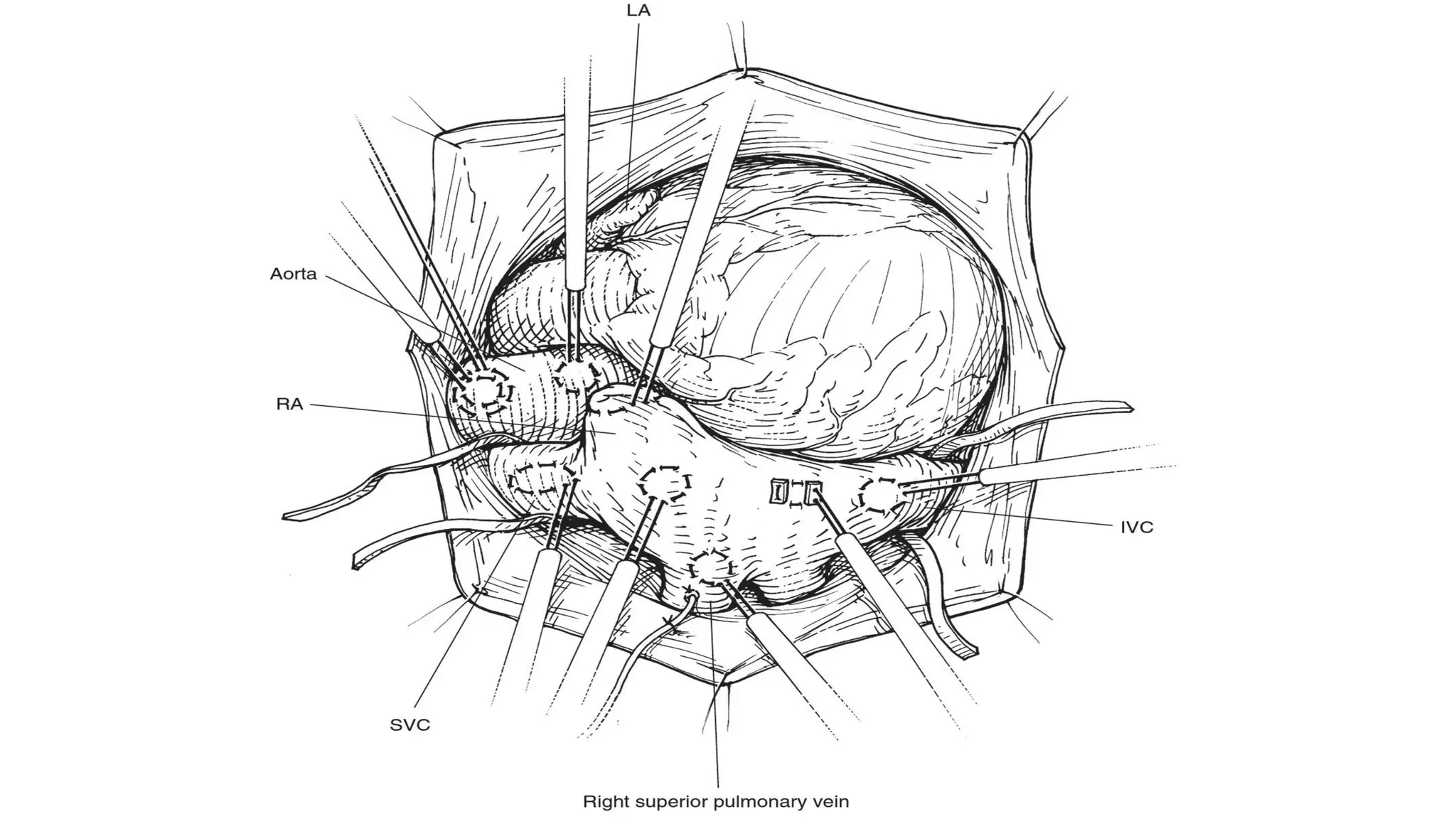
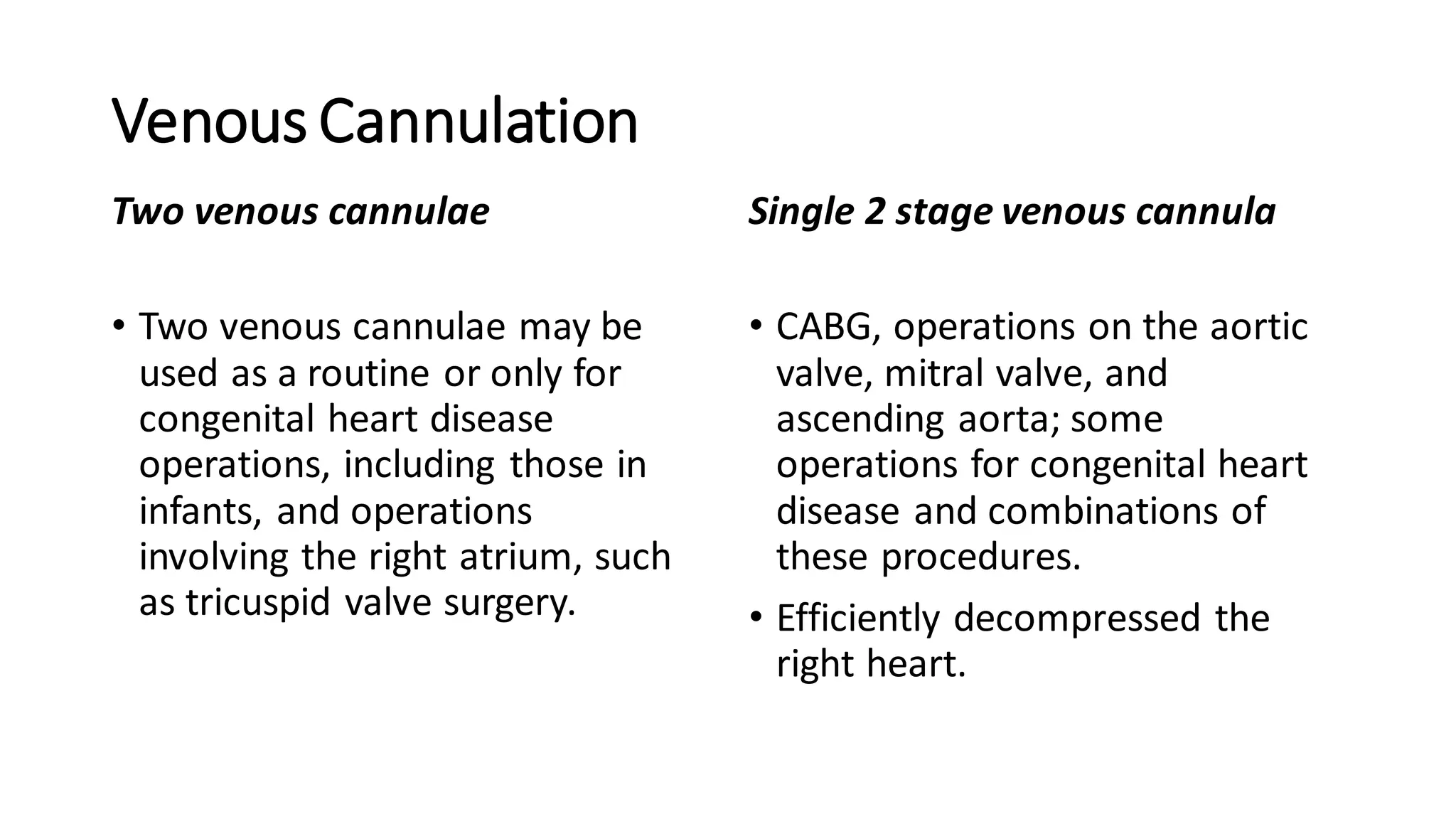
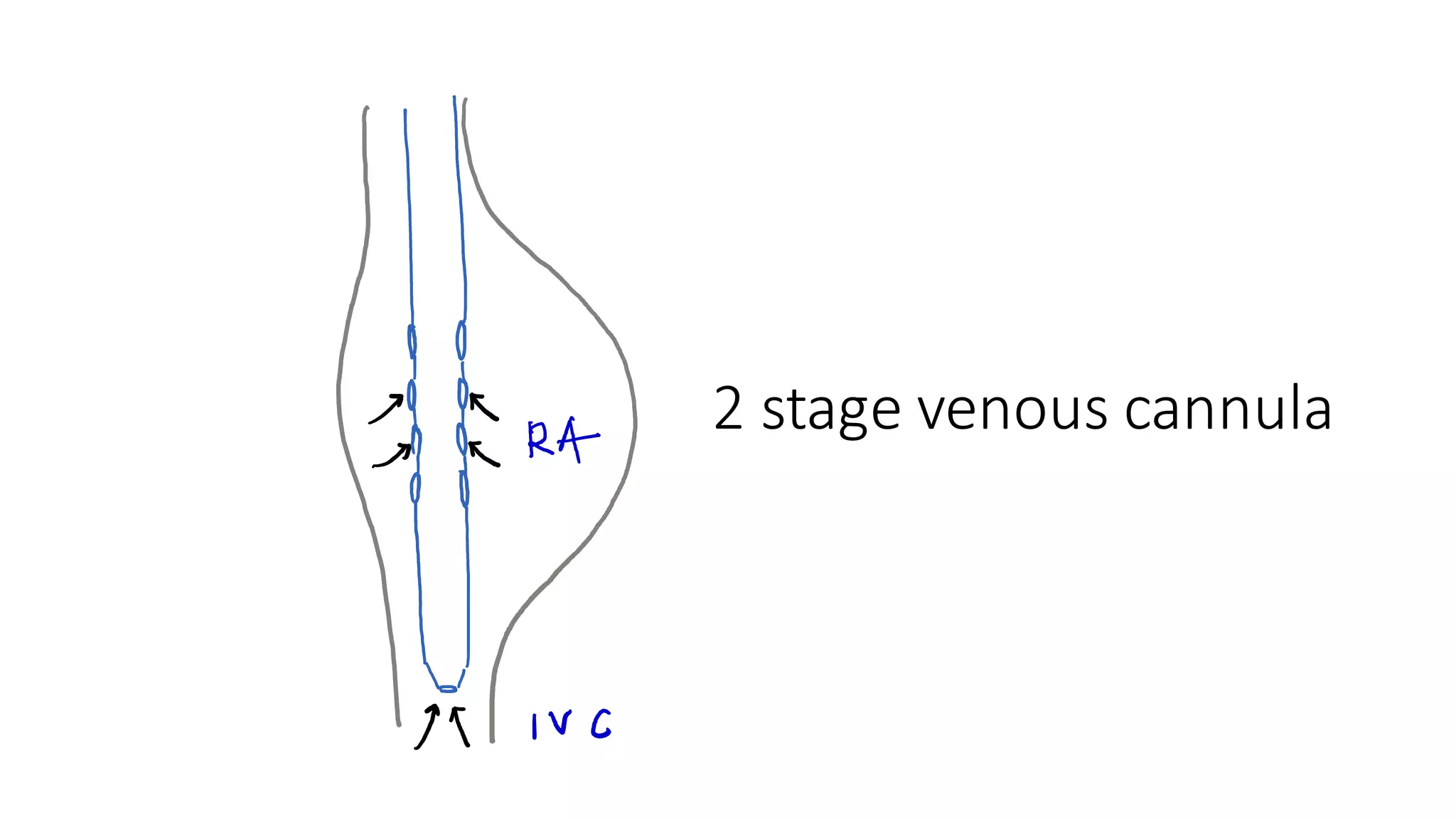
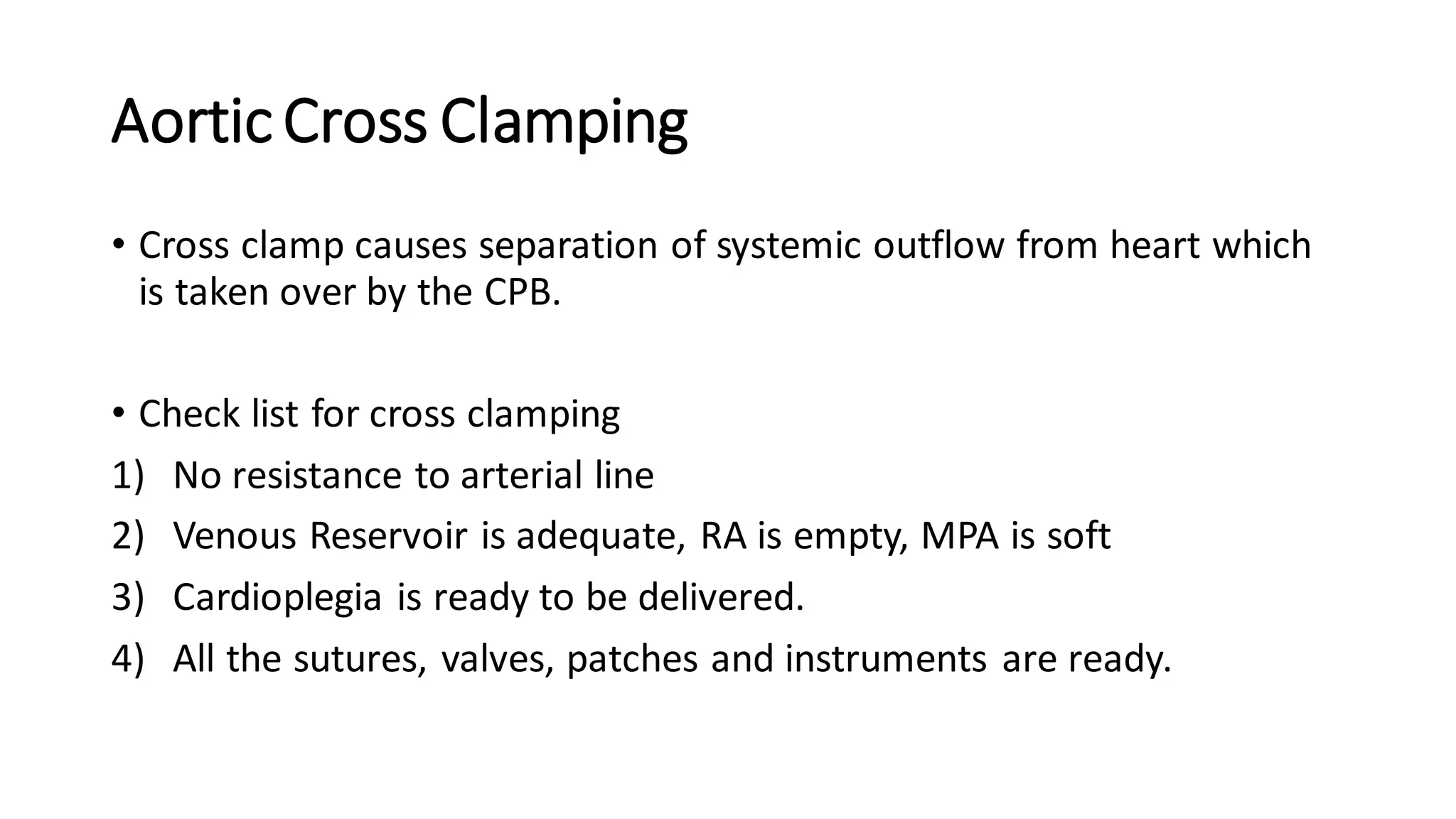
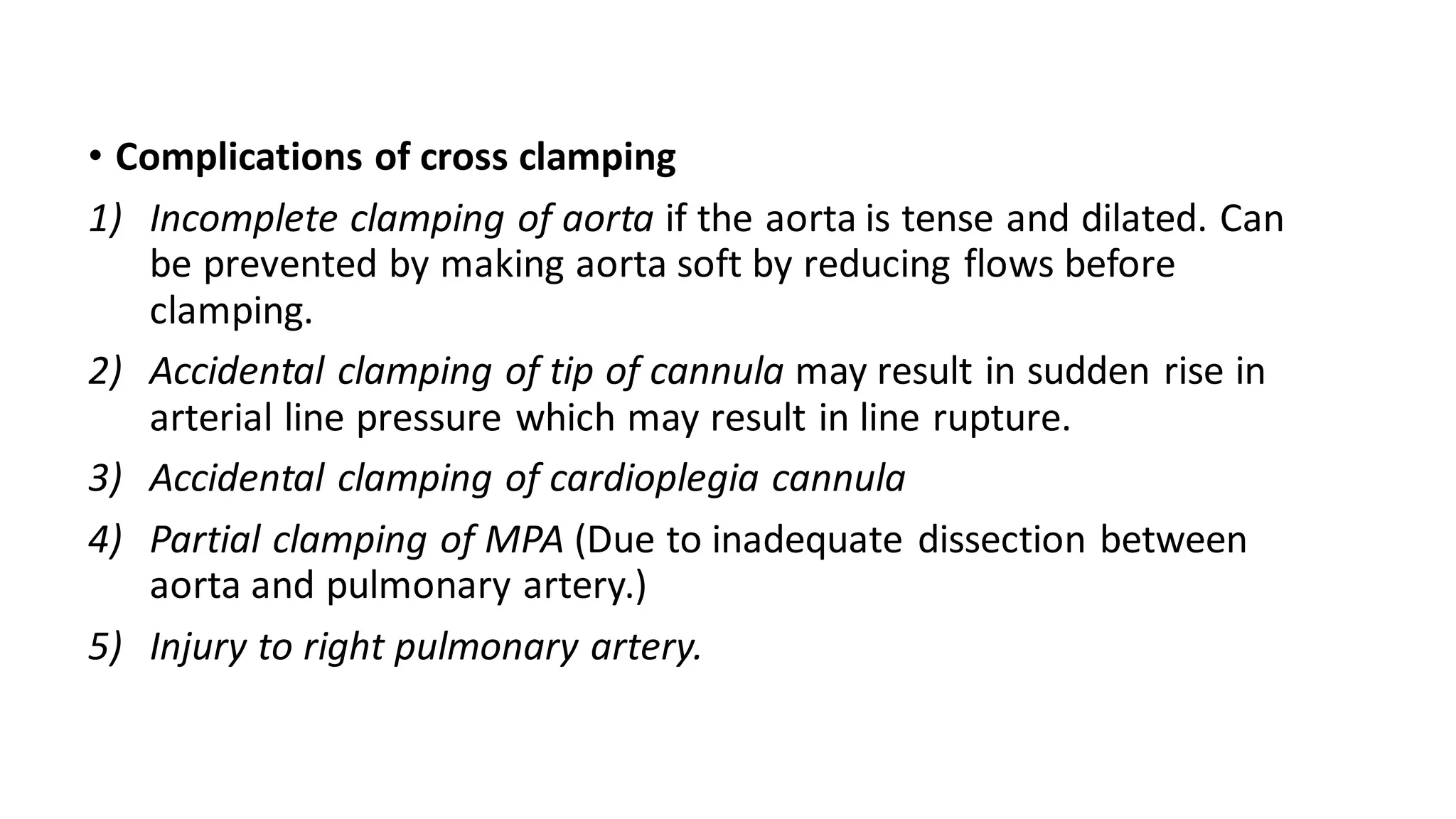
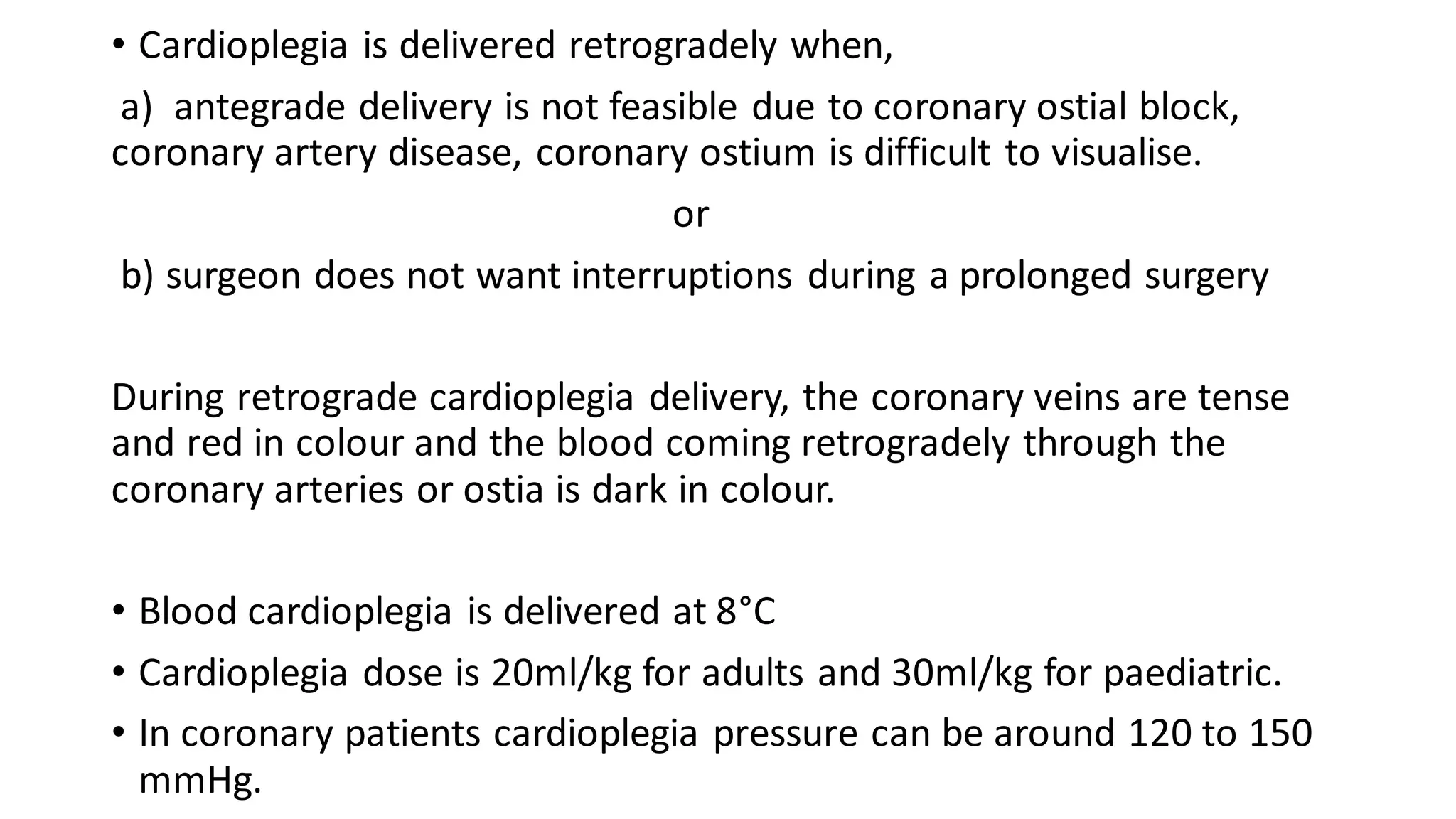
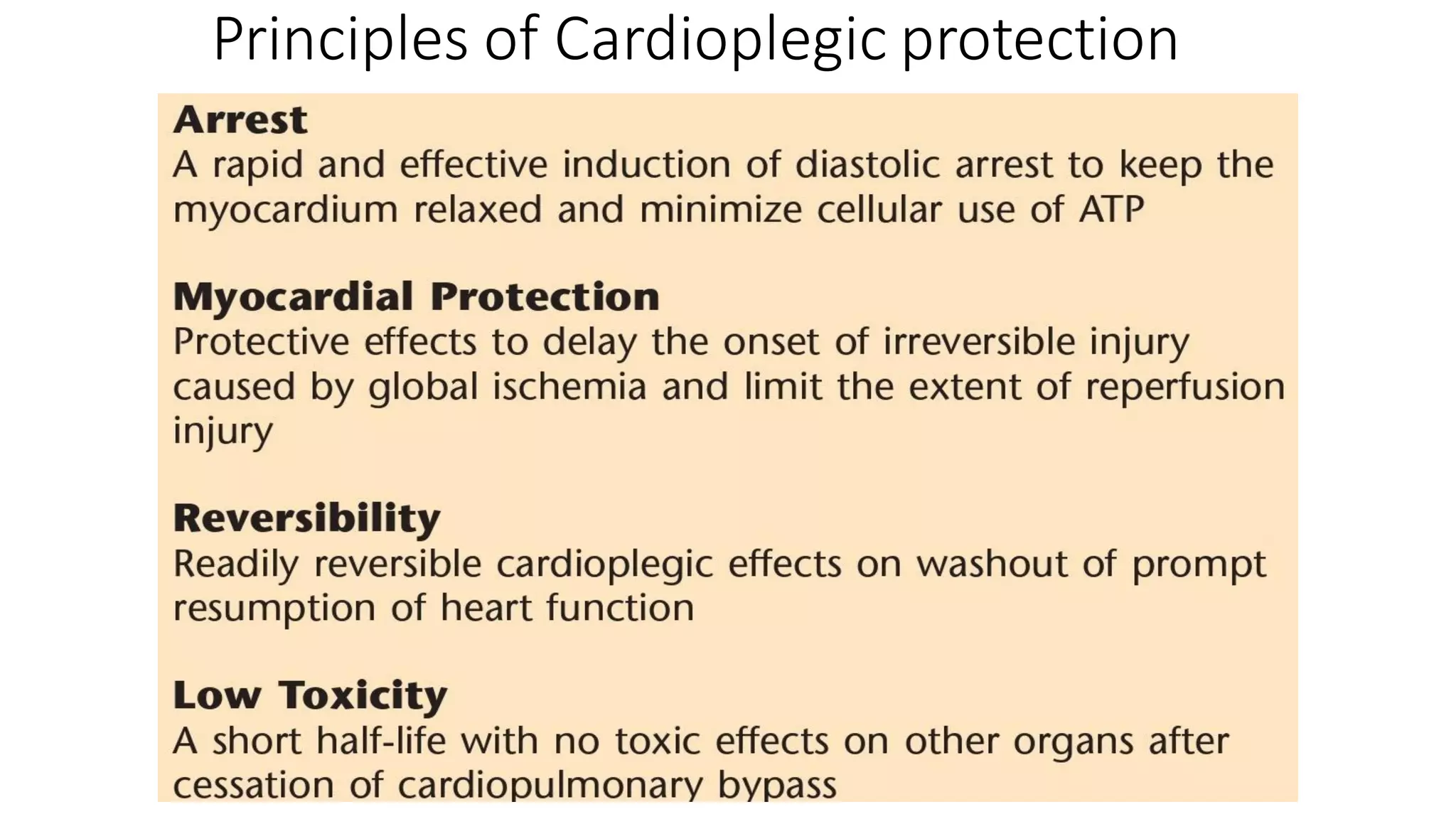
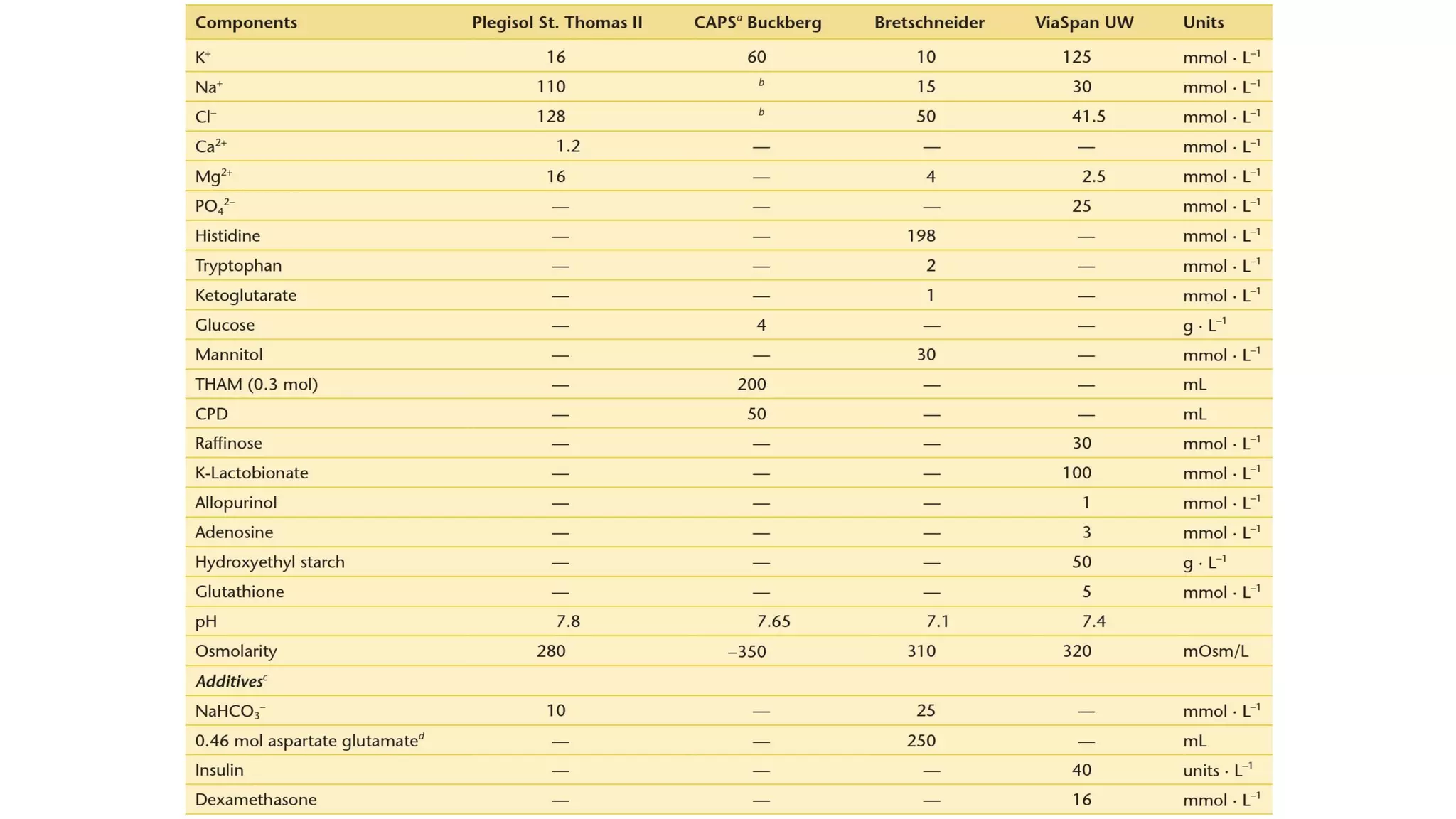
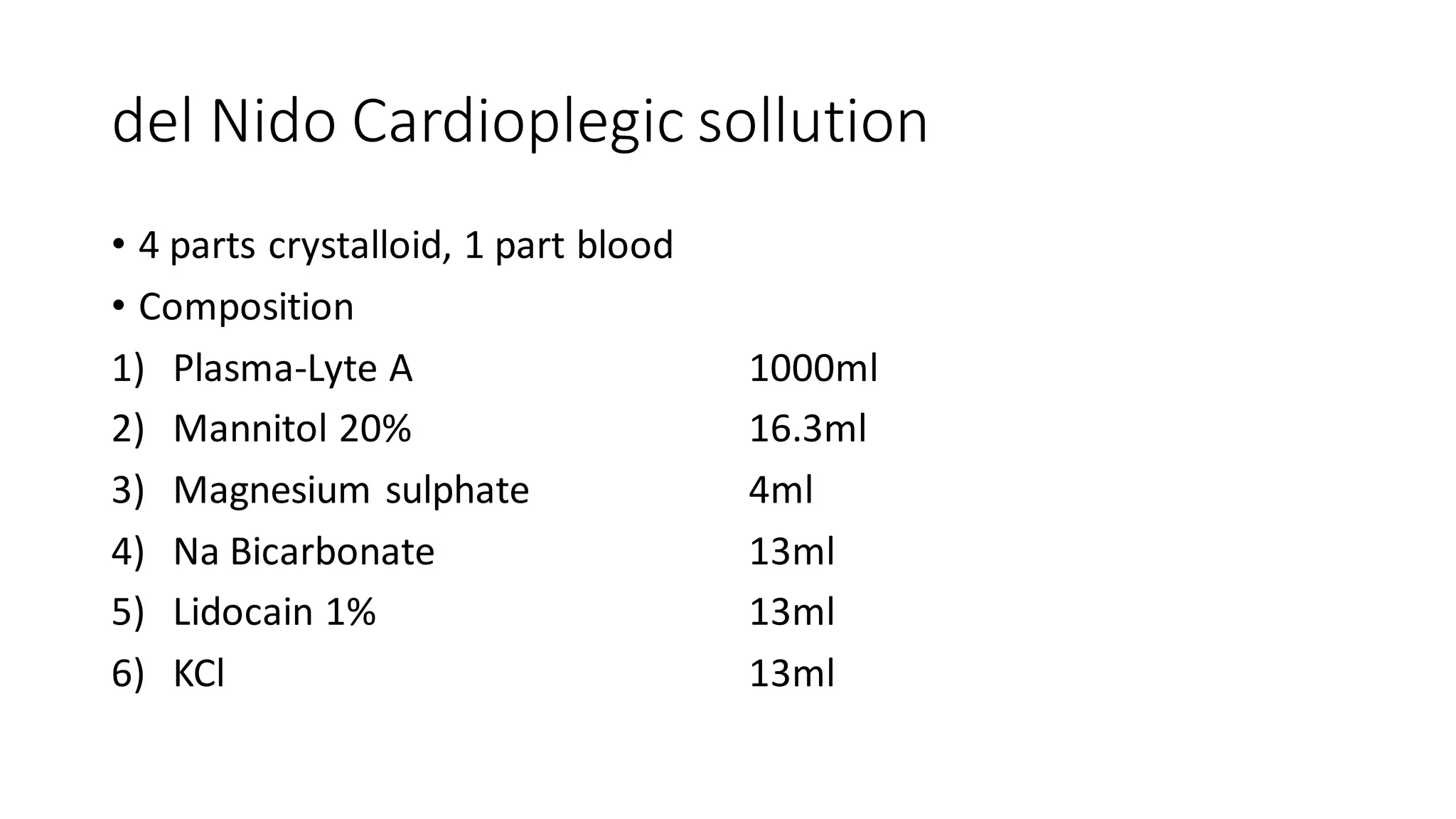
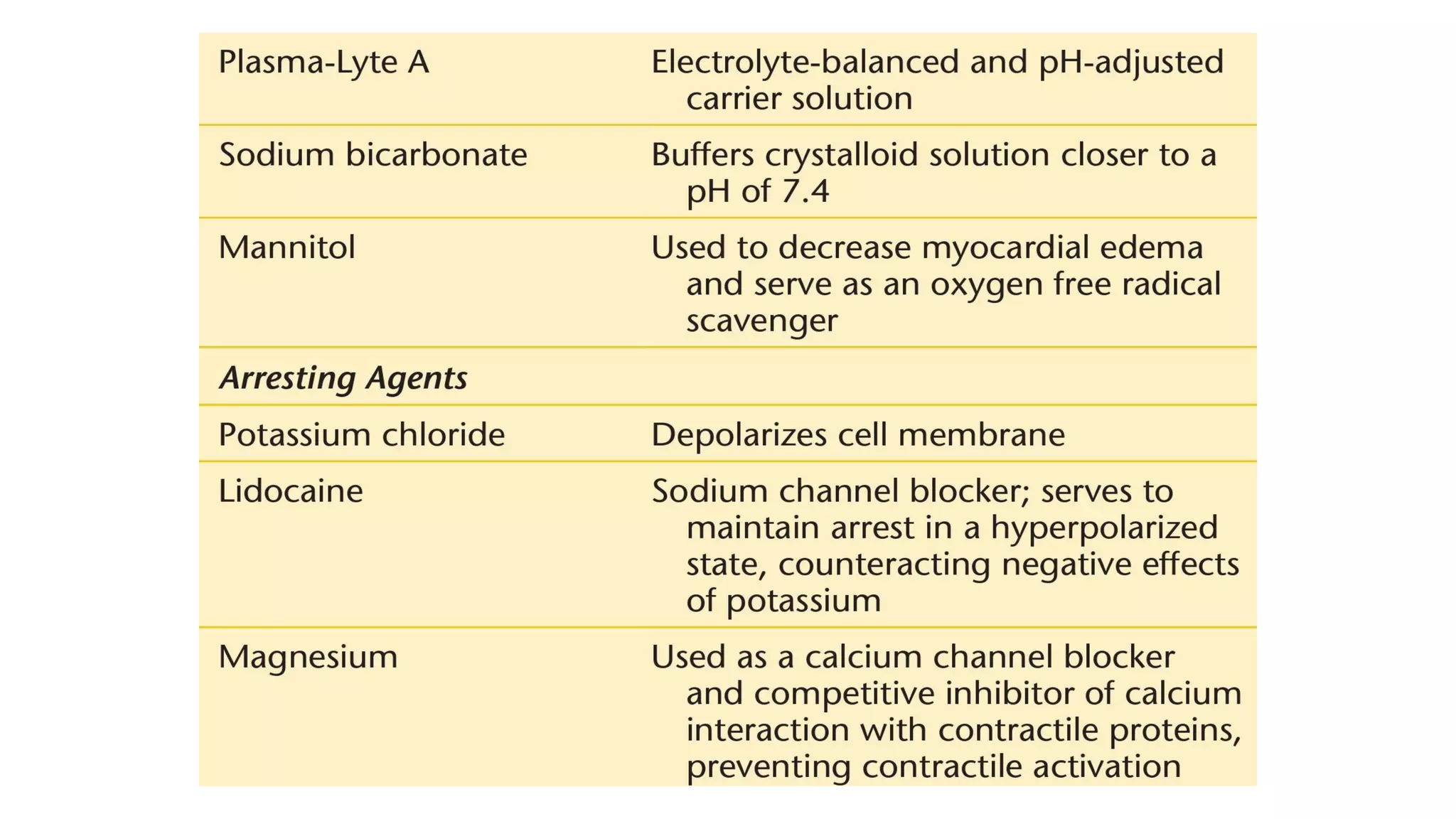
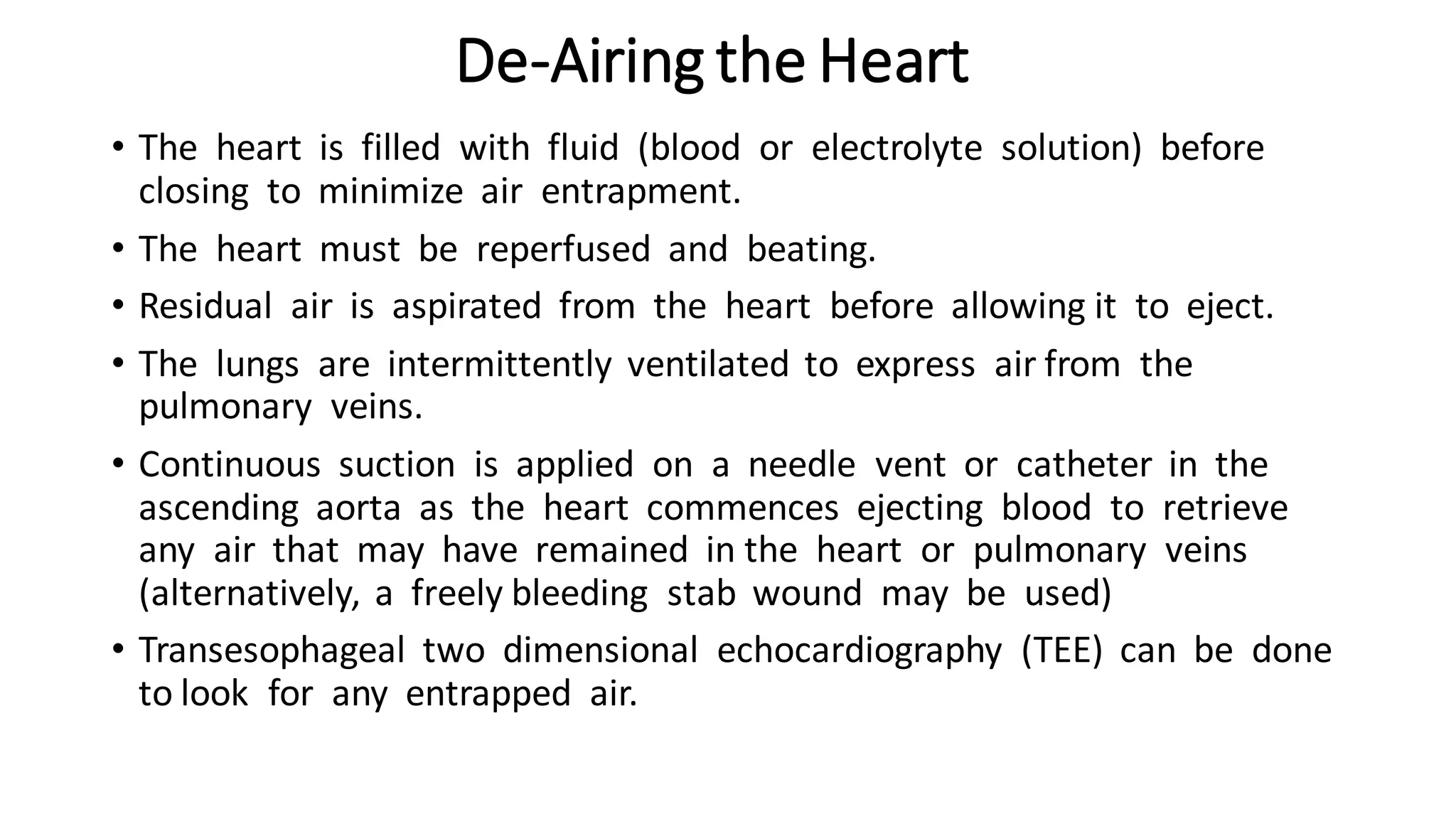
- CPB allows for an open, bloodless field during cardiac surgery by taking over the functions of the heart and lungs. Various techniques like hypothermia, cardioplegia, and venting are used to protect the heart during bypass.