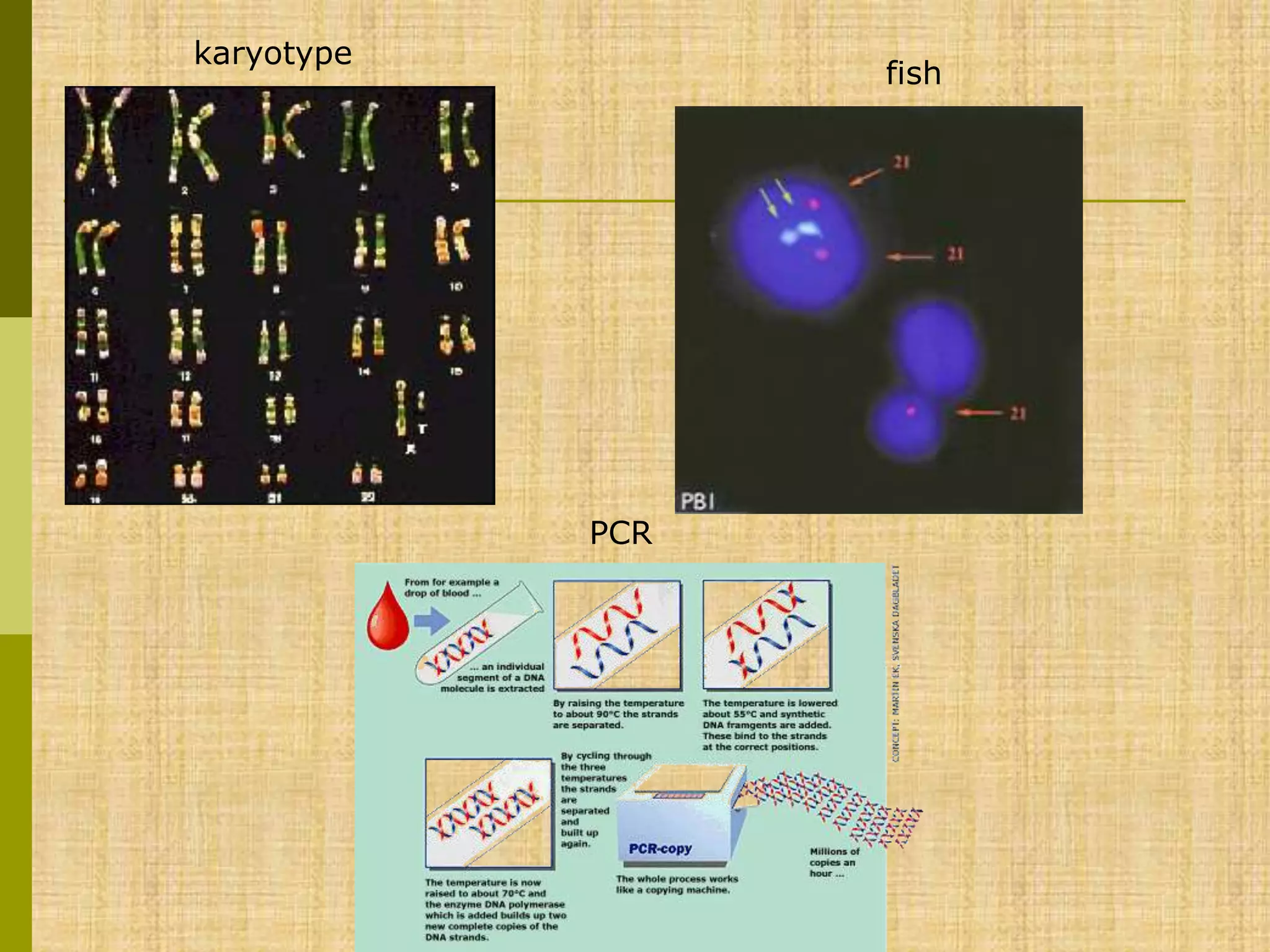
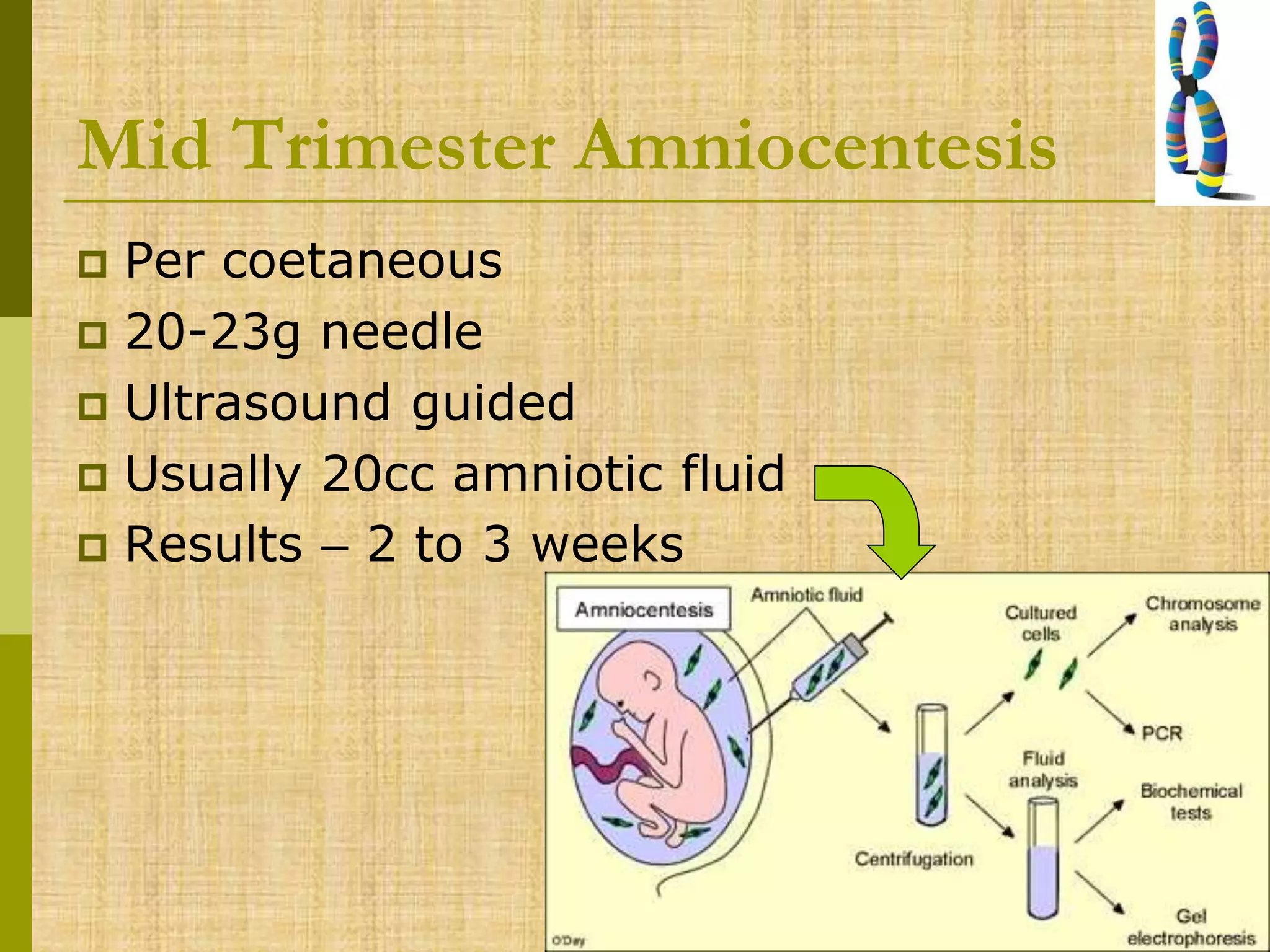
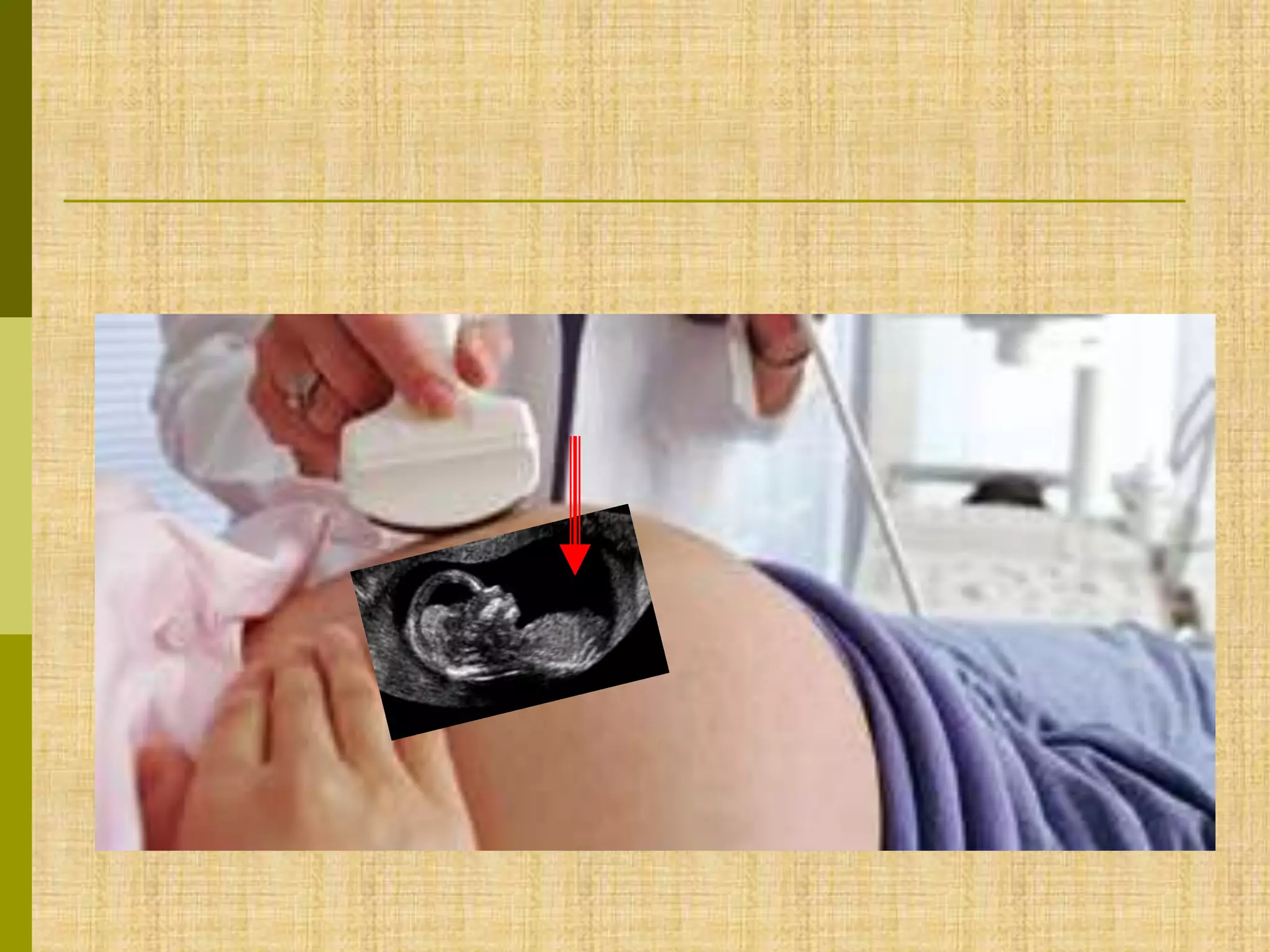
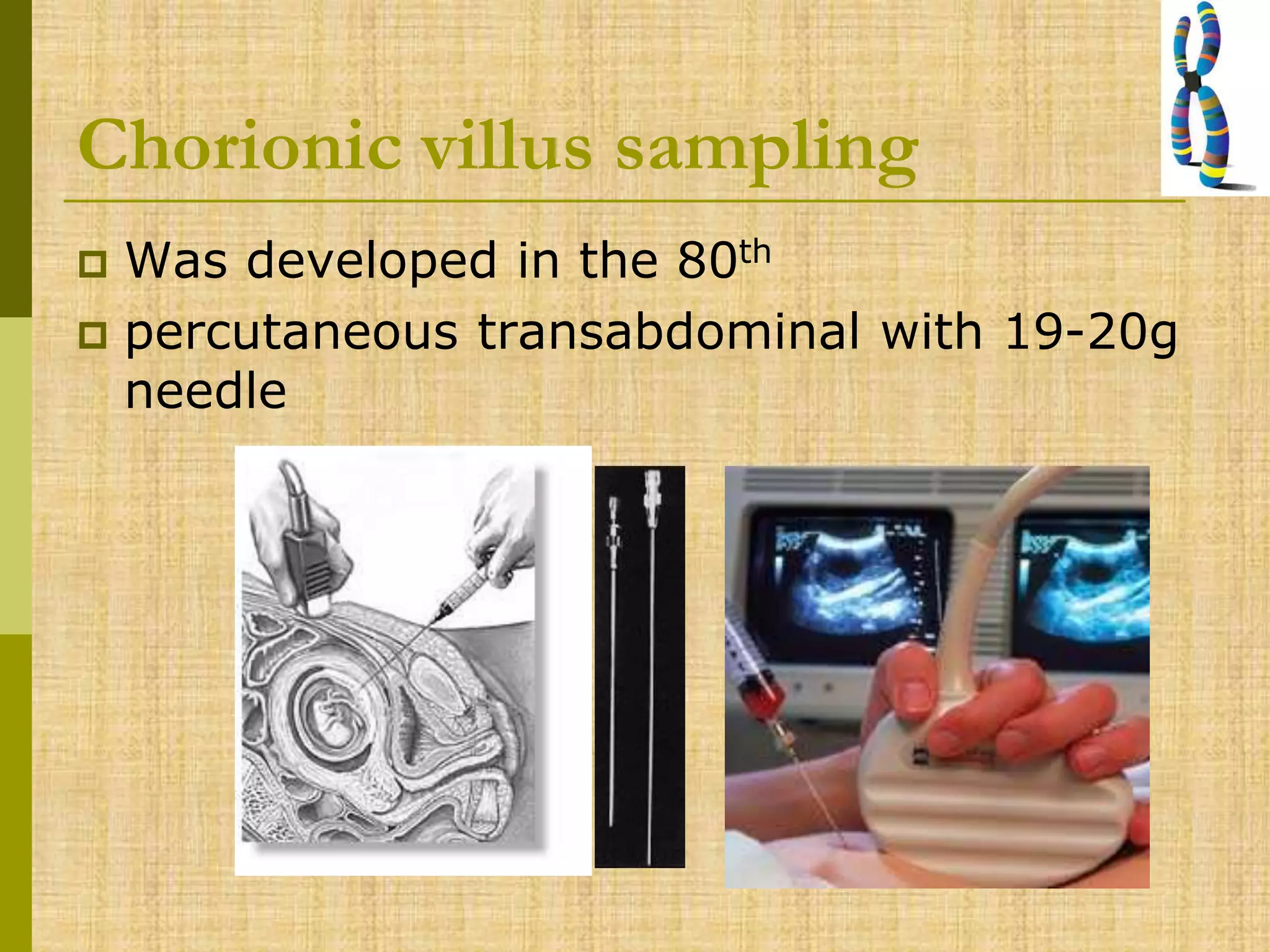
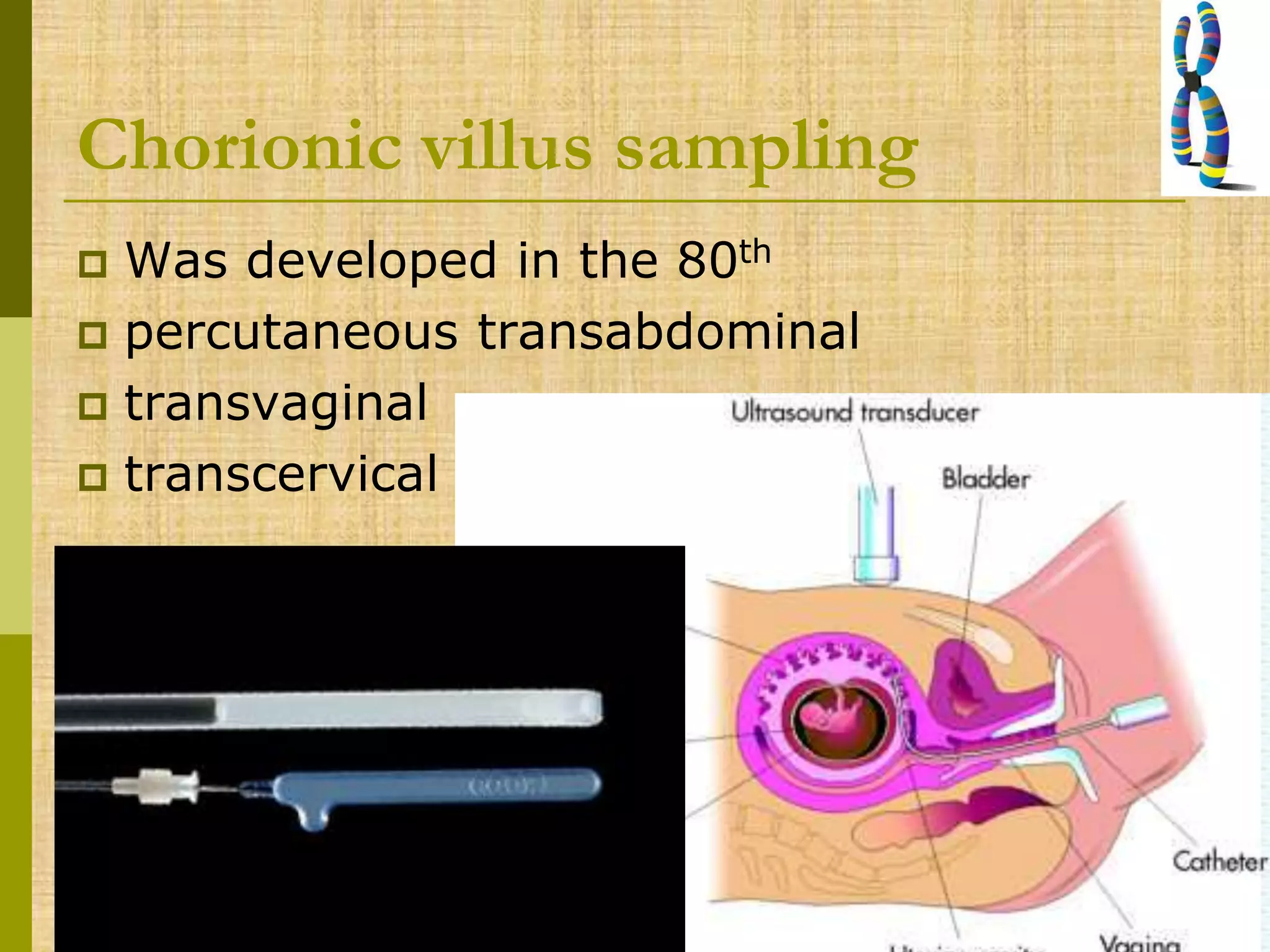
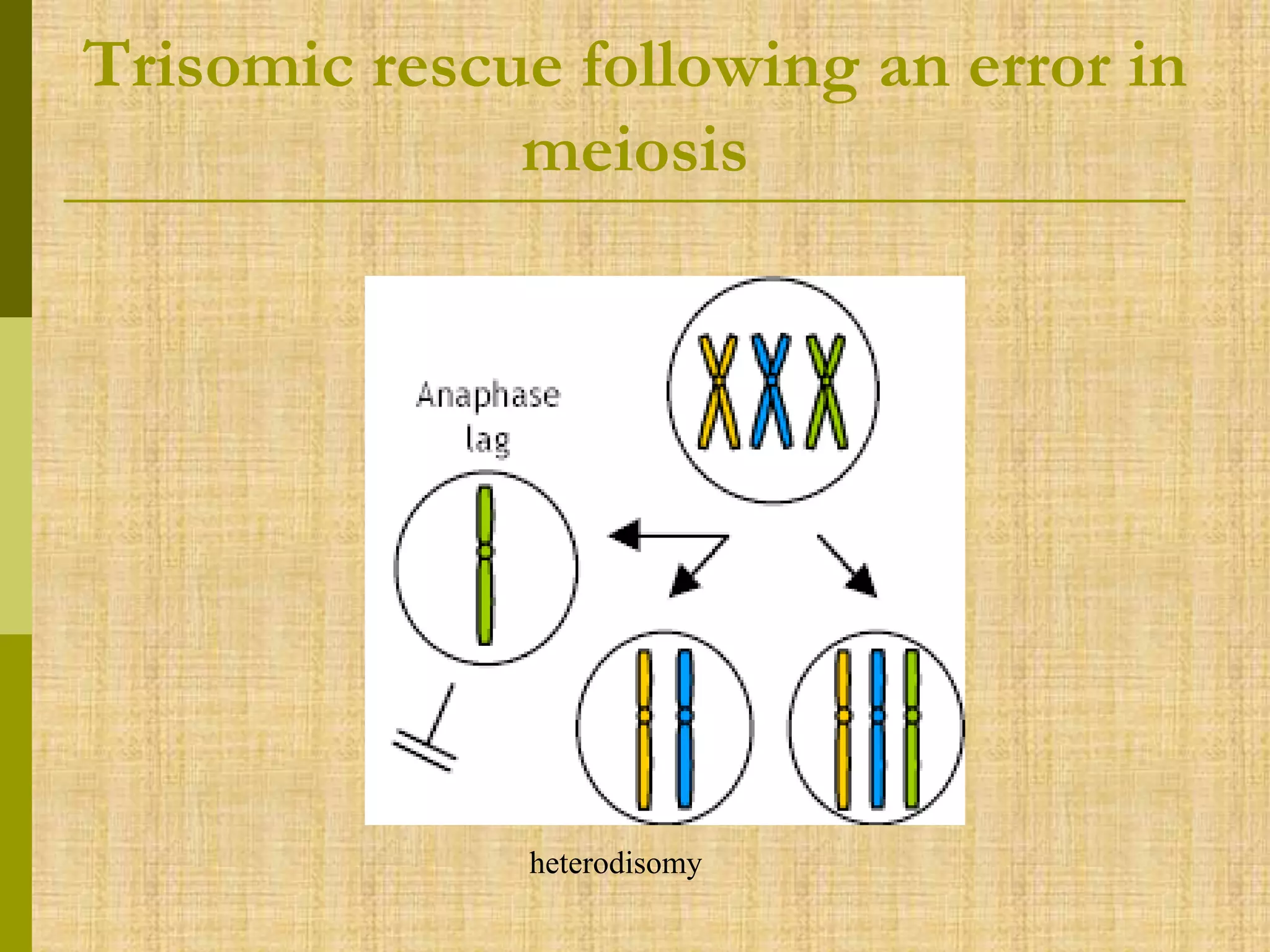
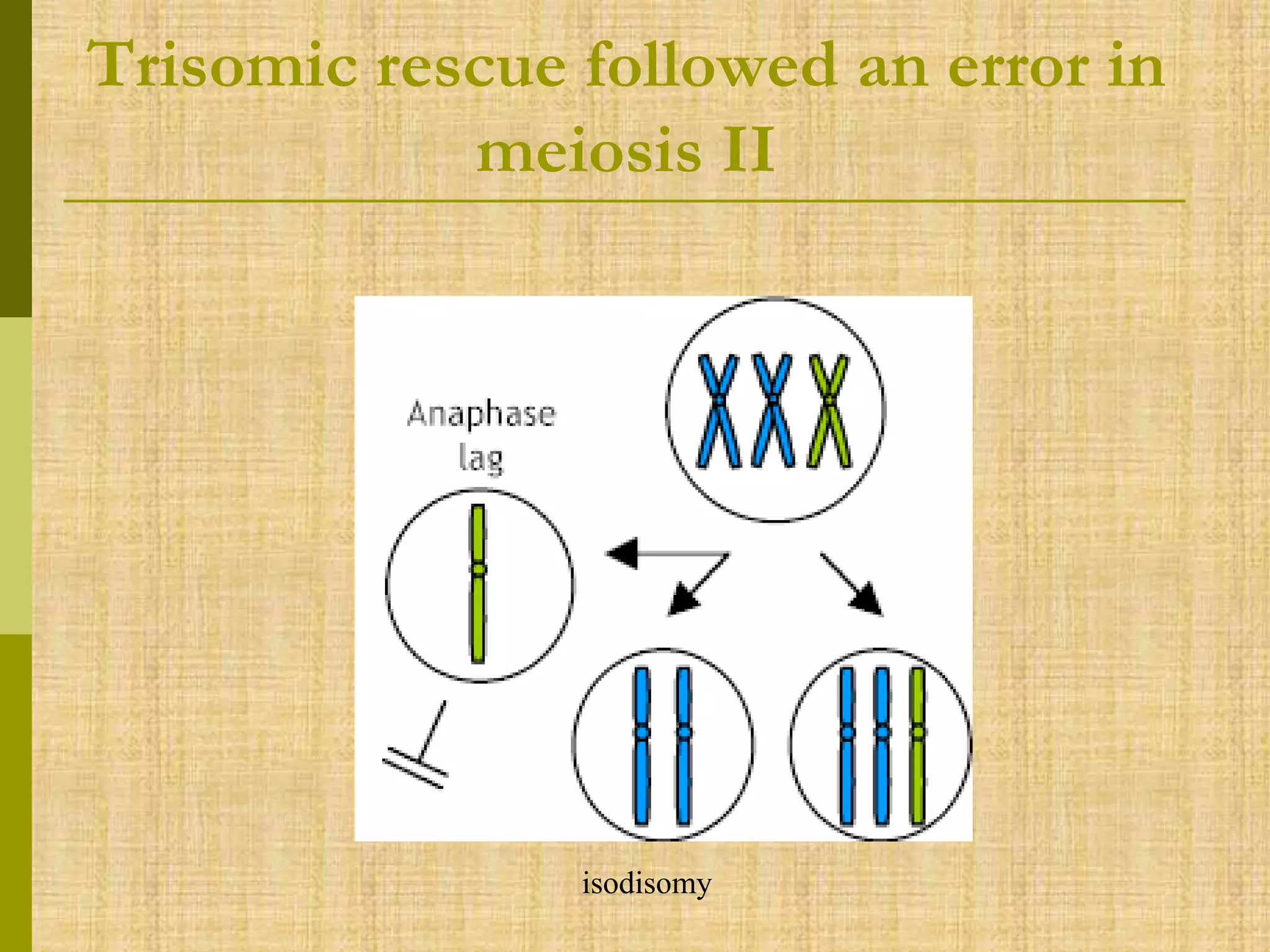
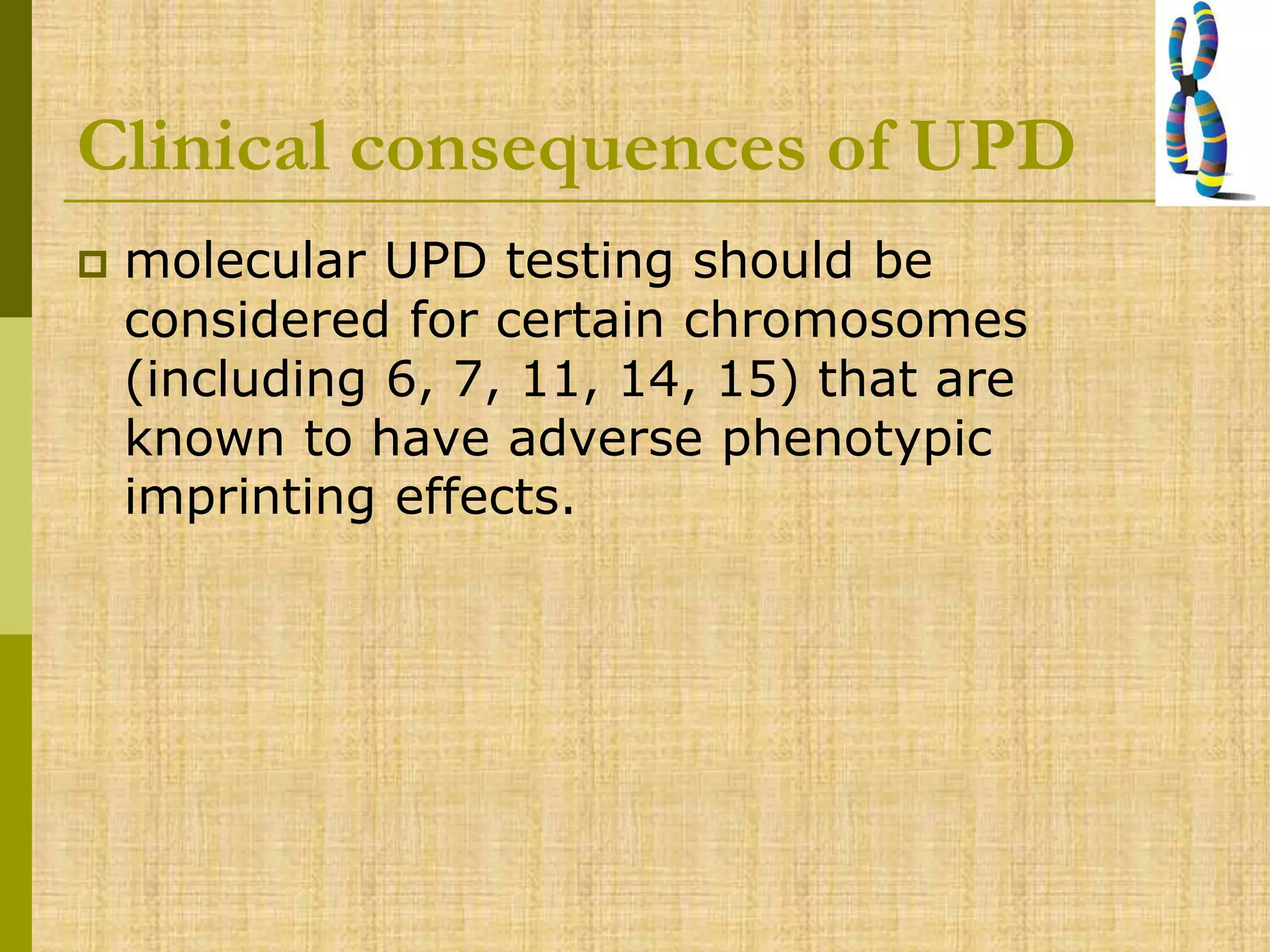
The document discusses methods for chromosomal evaluation during pregnancy, including both non-invasive (fetal cells from maternal blood) and invasive techniques (amniocentesis and chorionic villus sampling). It highlights the risks and complications associated with these methods, such as pregnancy loss and chromosomal abnormalities, and reviews factors influencing the outcomes of mosaicism and uniparental disomy. Additionally, it provides guidelines for prenatal diagnostics and the implications of various chromosomal findings.