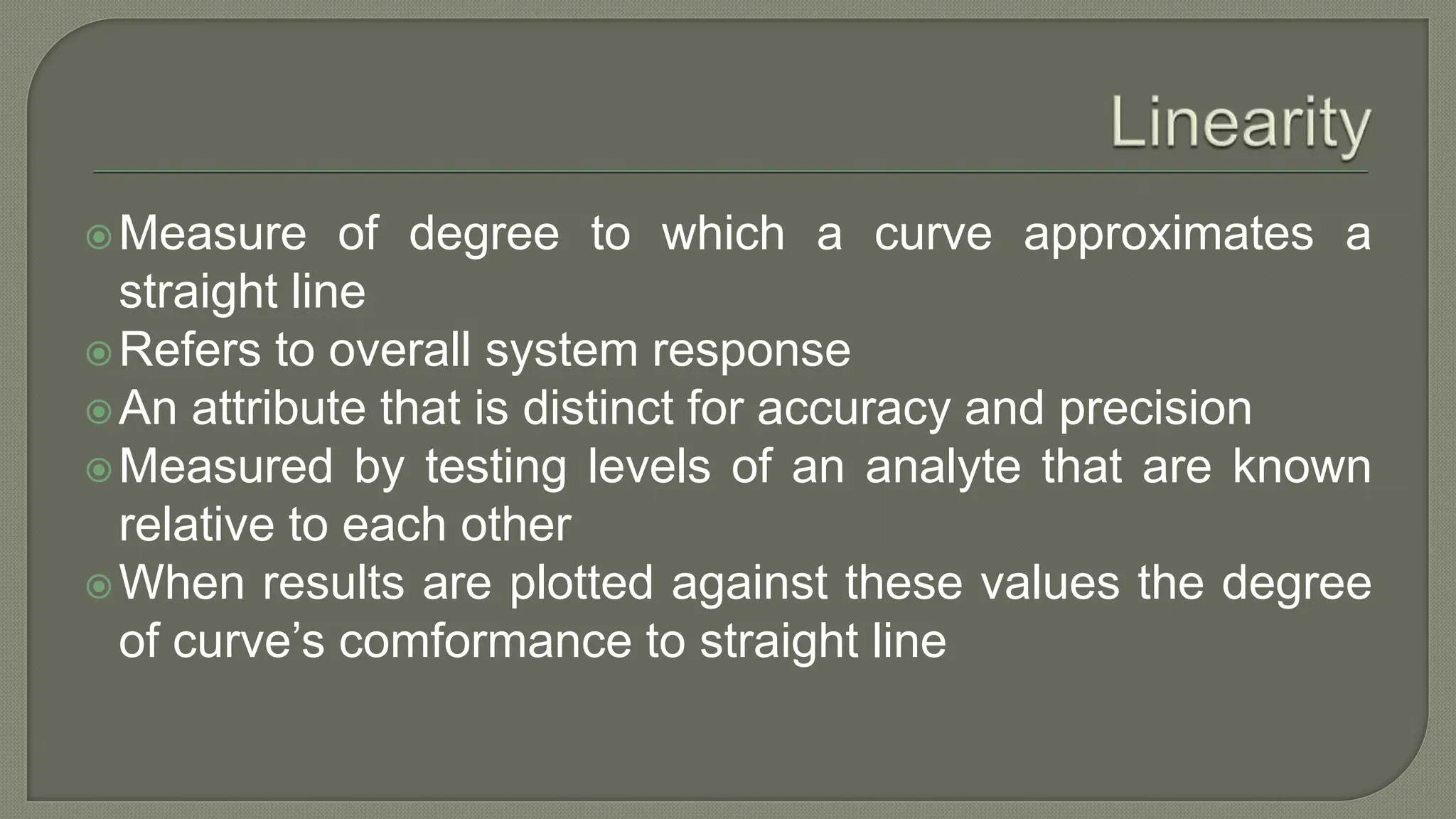
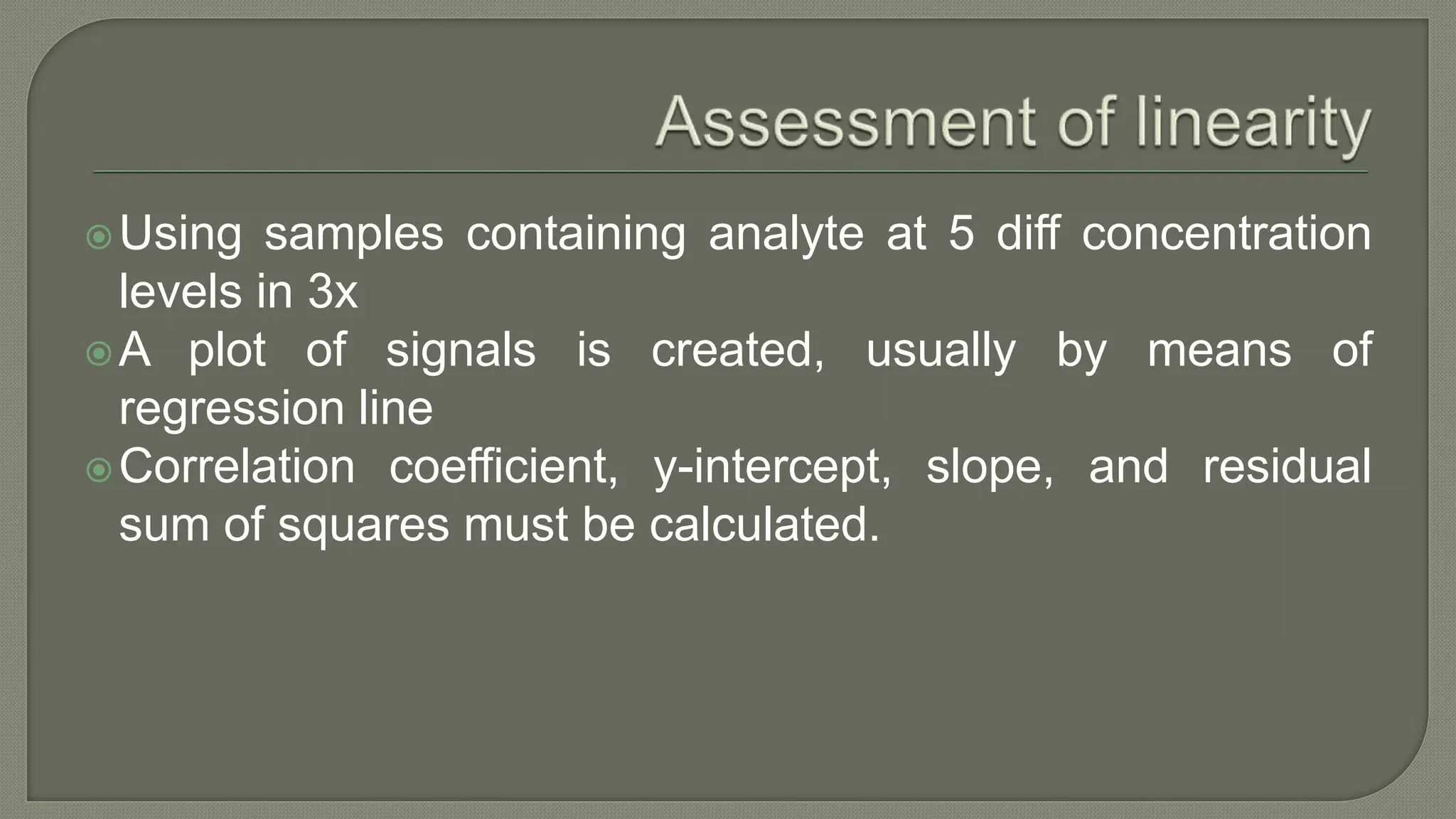
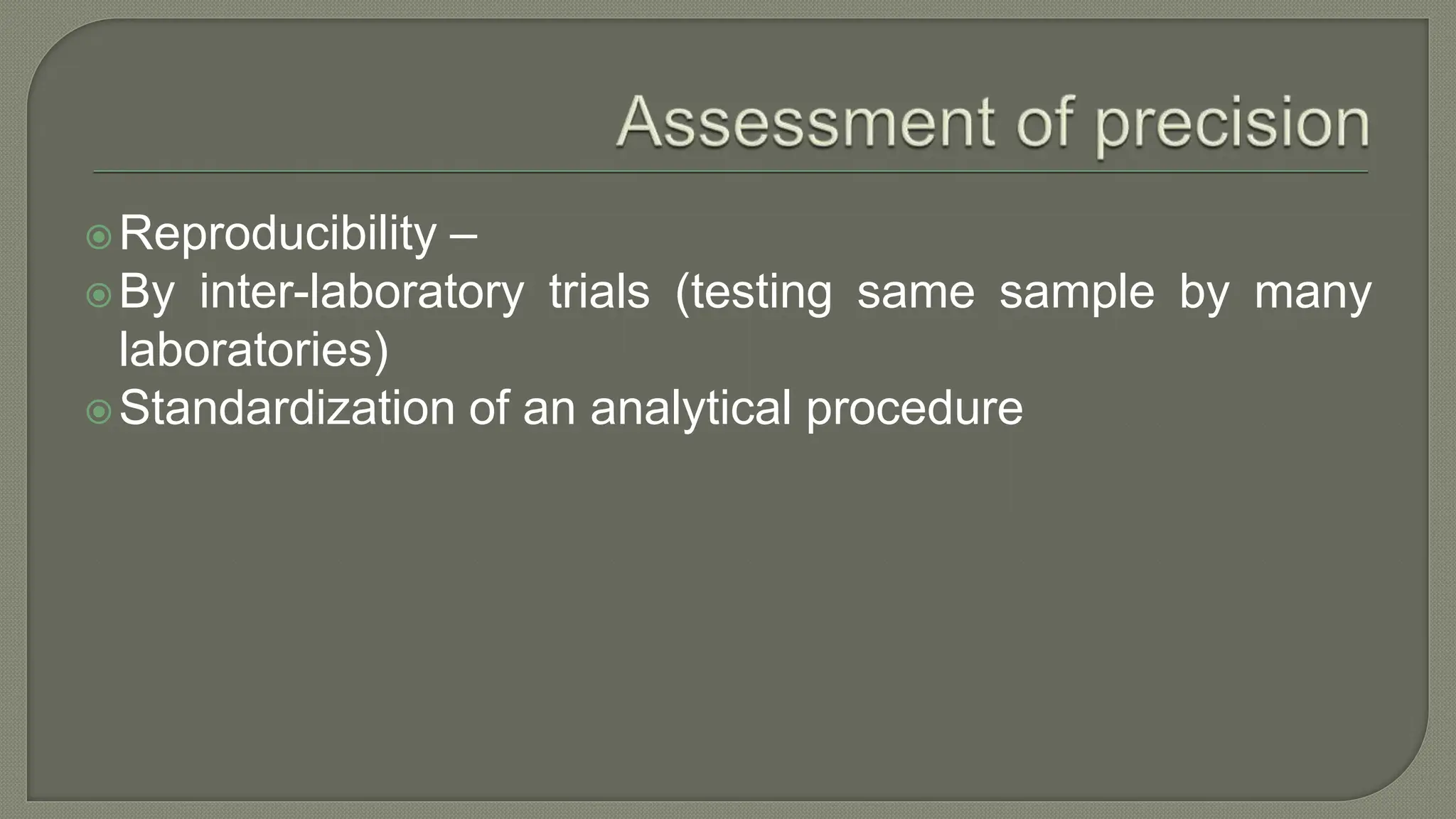
The document outlines the essential steps for method selection in analytical biochemistry, focusing on the evaluation of performance characteristics such as precision, accuracy, analytical range, detection limit, and specificity. It emphasizes the importance of method verification, validation, and quality control practices in laboratory settings, along with the necessity of establishing a startup phase for troubleshooting and training. Additionally, it discusses the analytical procedures for determining the accuracy and precision of methods through comparative analyses and statistical measures.