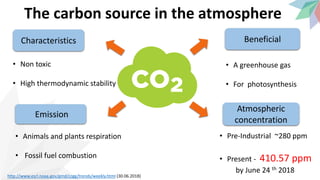
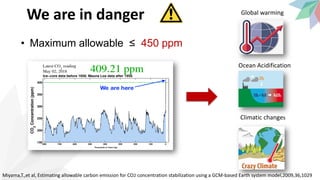
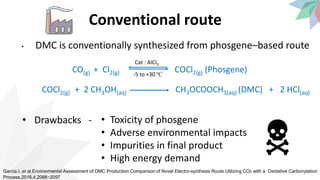
1) Atmospheric CO2 levels have risen from 280 ppm pre-industrially to over 410 ppm currently due to emissions from fossil fuel combustion and respiration. Maximum safe levels are believed to be 450 ppm or less to avoid worst effects of global warming and ocean acidification.
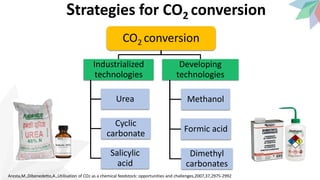
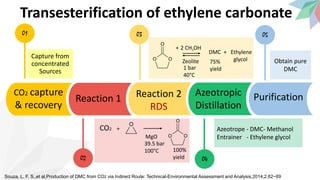
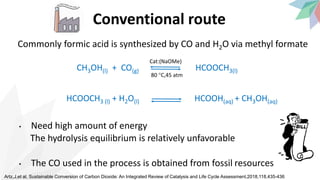
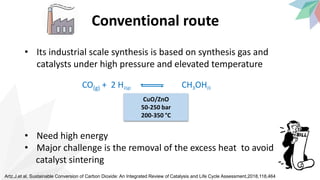
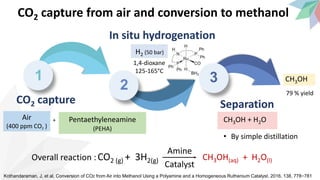
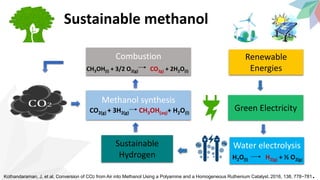
2) The document discusses strategies for converting CO2 into useful products like dimethyl carbonate (DMC), formic acid and methanol. It outlines more sustainable routes for producing these chemicals directly from CO2 rather than traditional methods that rely on other carbon sources.
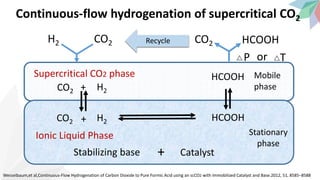
3) Specifically, it presents a method for continuously producing pure formic acid by hydrogenating supercritical CO2 with an immobilized catalyst and base, avoiding high