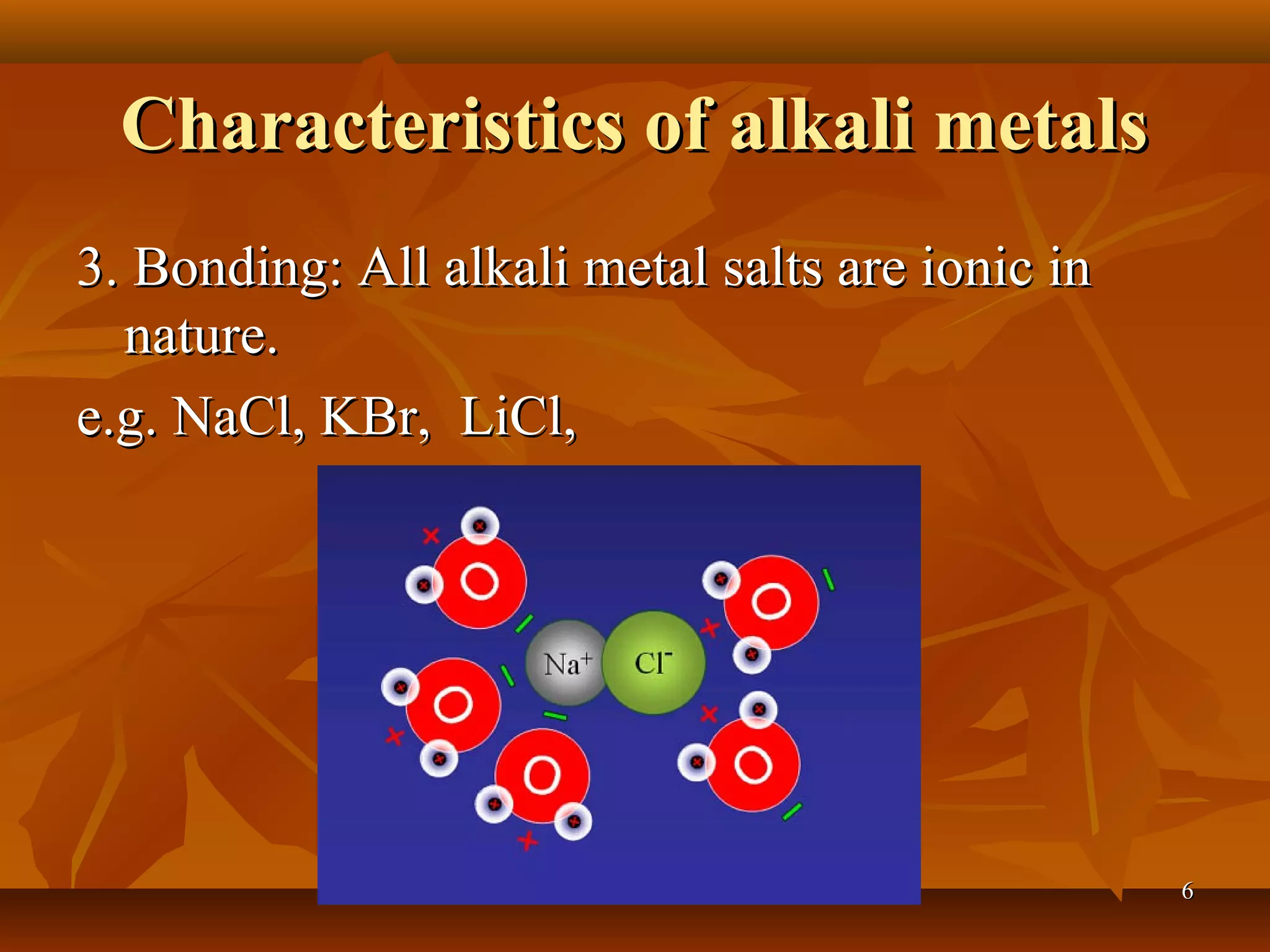
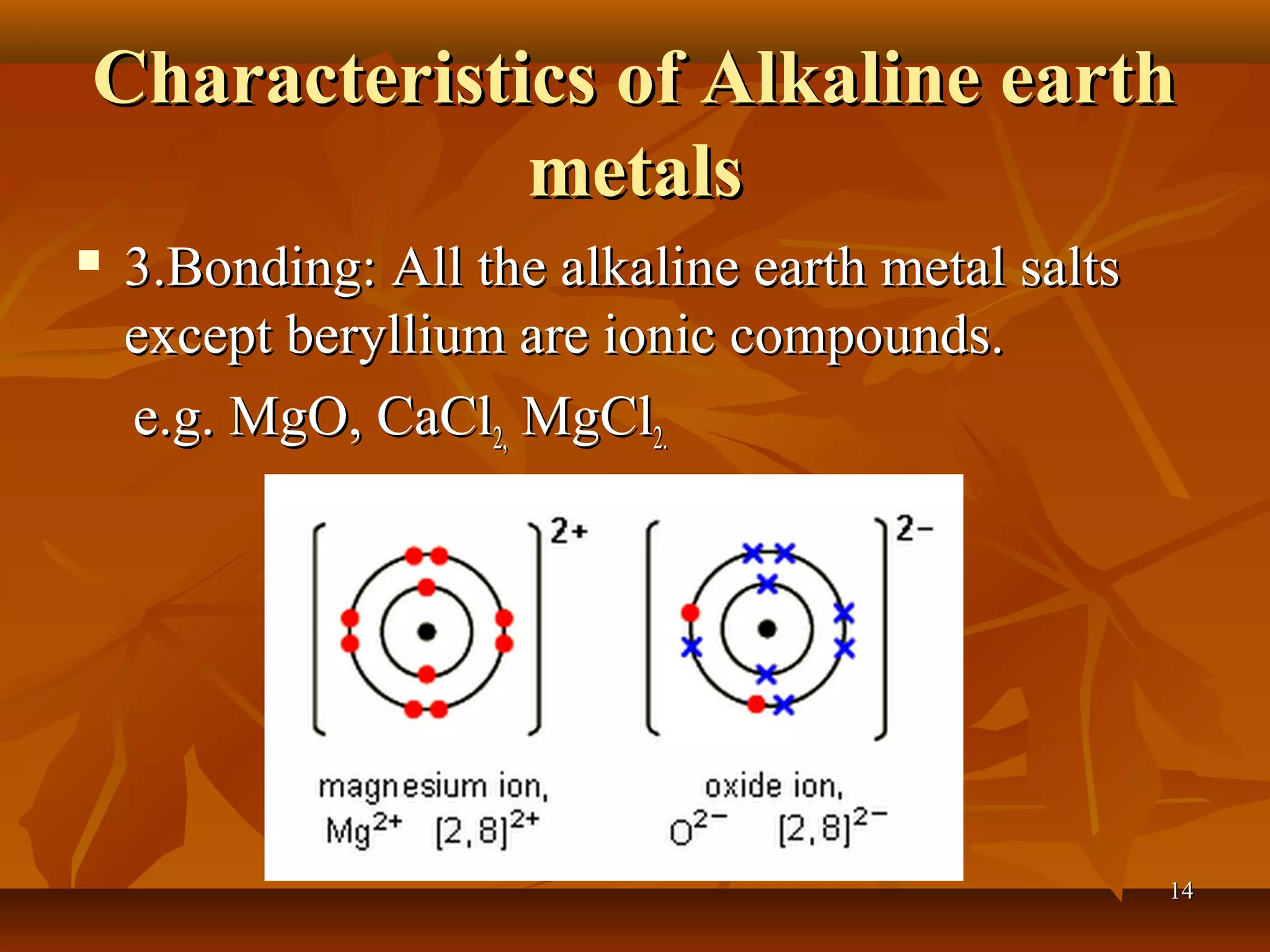
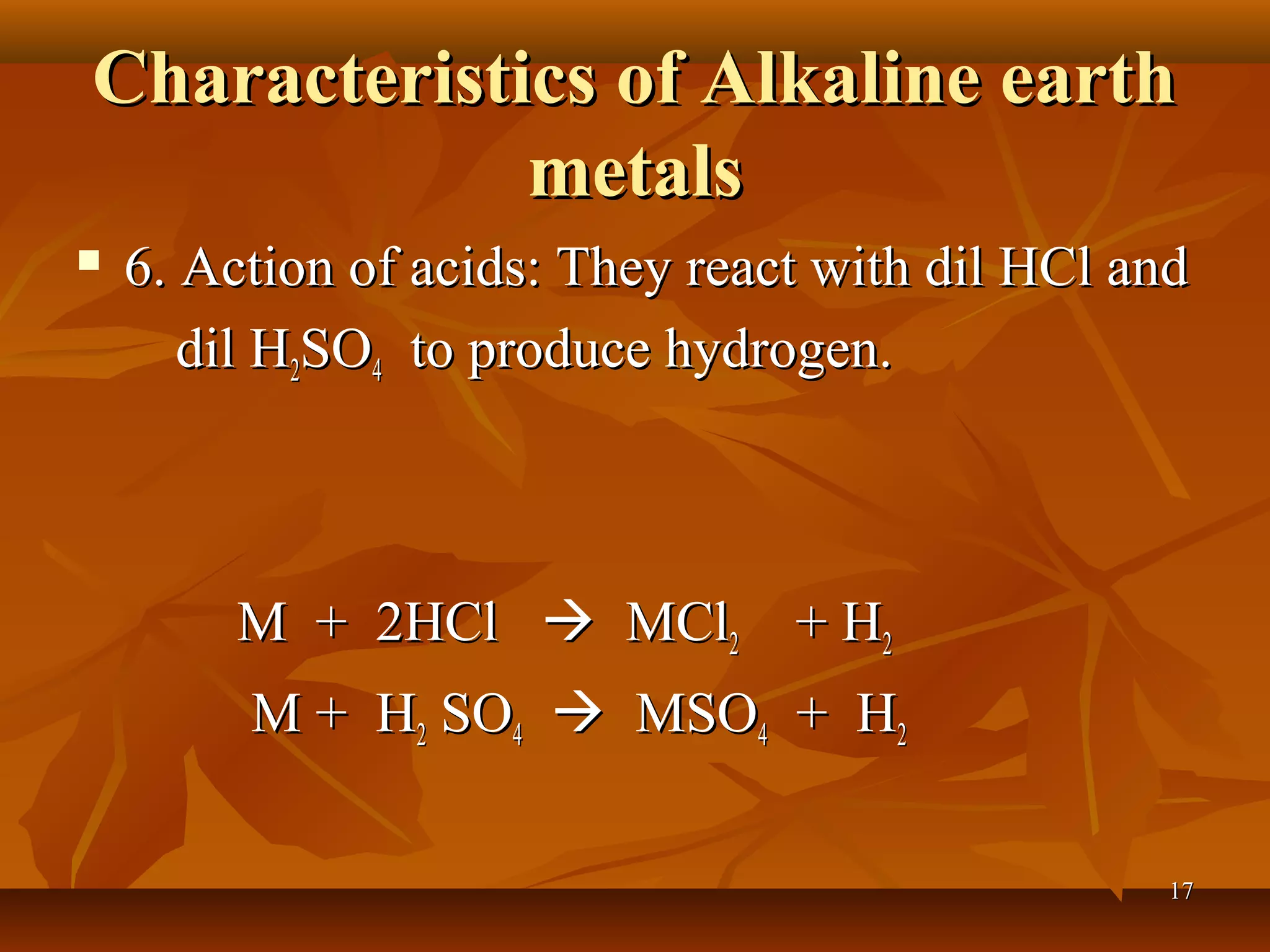
The document discusses alkali metals and alkaline earth metals. Alkali metals such as lithium, sodium, and potassium are placed in Group 1A of the periodic table and have one electron in their outermost shell. They react violently with water to form strong alkali hydroxides and hydrogen gas. Alkaline earth metals like beryllium, magnesium, and calcium are in Group 2A and have two outer electrons. They are less reactive than alkali metals but still react with water and acids to produce hydrogen. The reactivity of both groups increases down the respective groups.