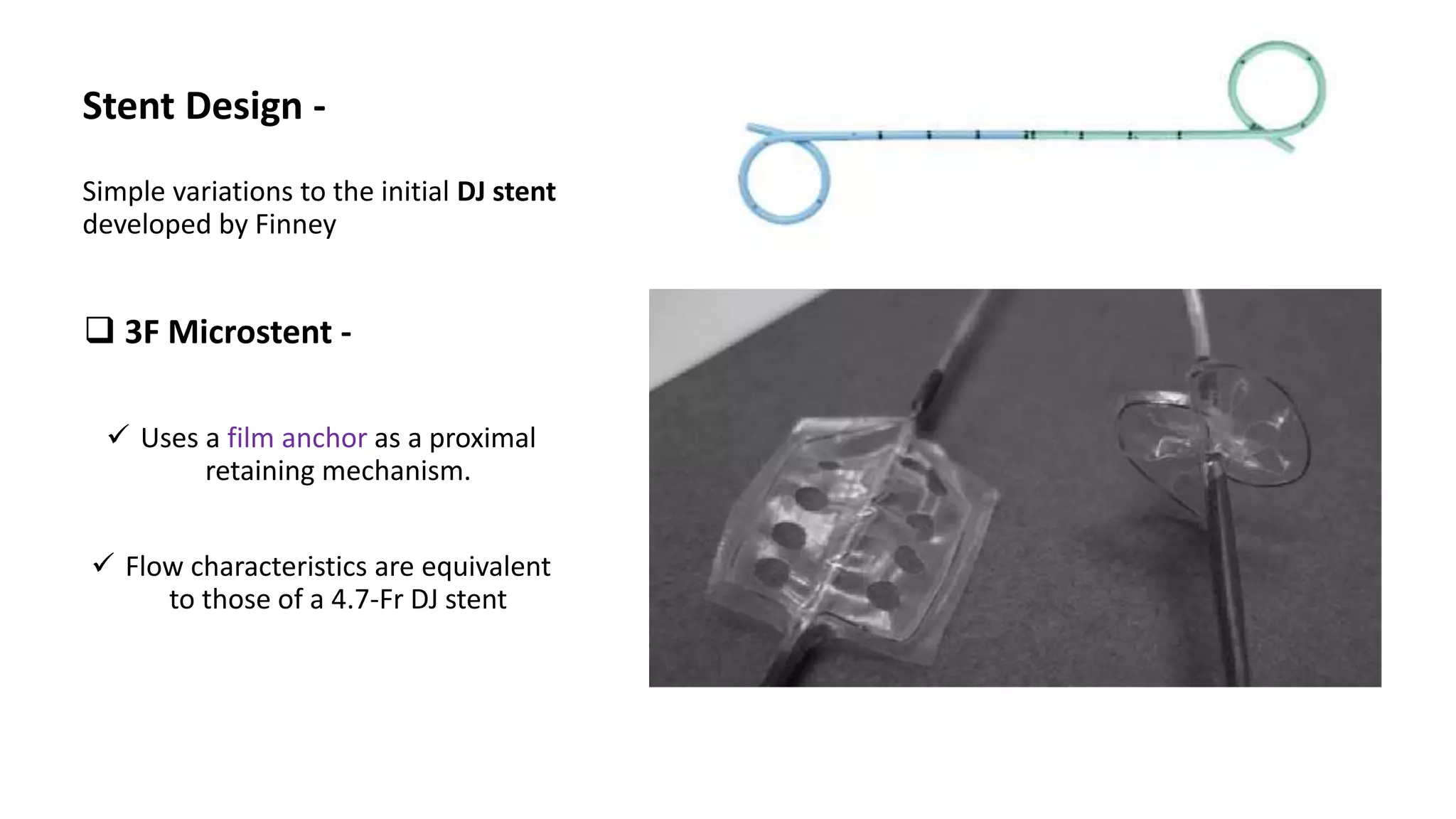
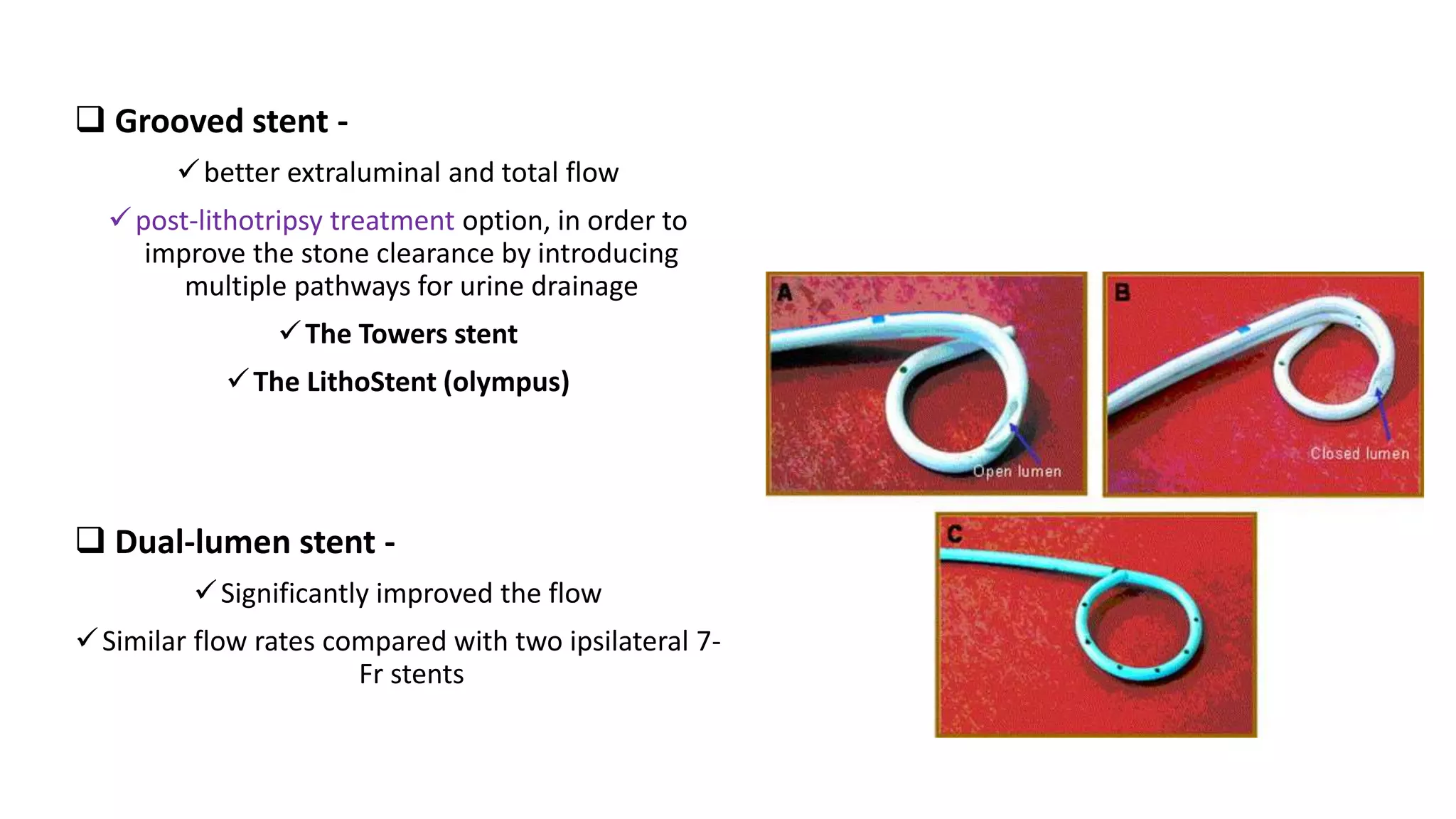
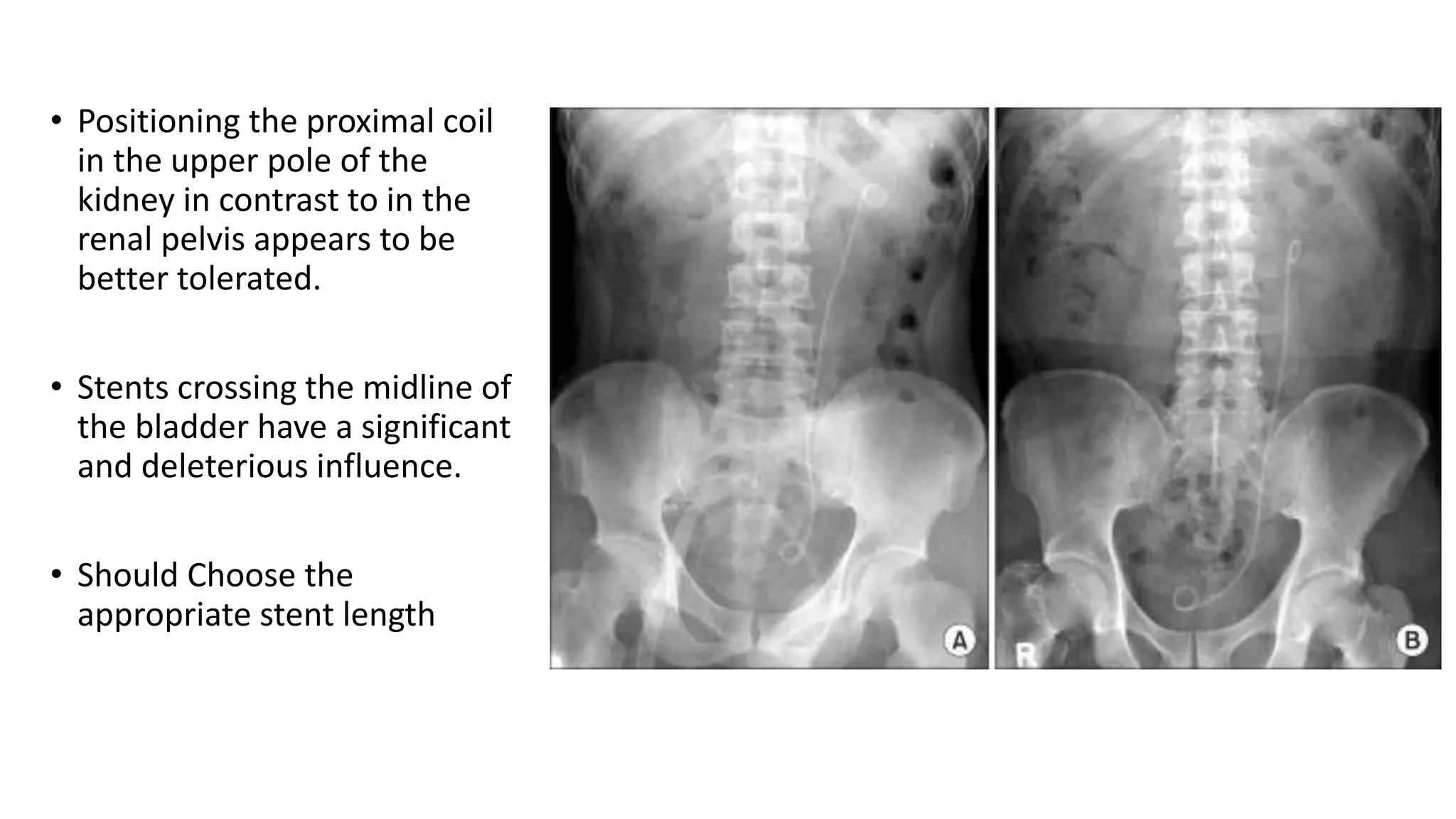
This document discusses ureteral stents used in urology. It provides a brief history of stent development and outlines ideal stent properties. Common stent materials like silicone, polyethylene and polyurethane are described. The document also discusses various stent designs, coatings, and indications for stent placement including for conditions like ureteral obstruction, urinary stone treatment, and transplantation. Complications are minimized by using the shortest possible indwelling time.