This document provides an overview of medical devices:
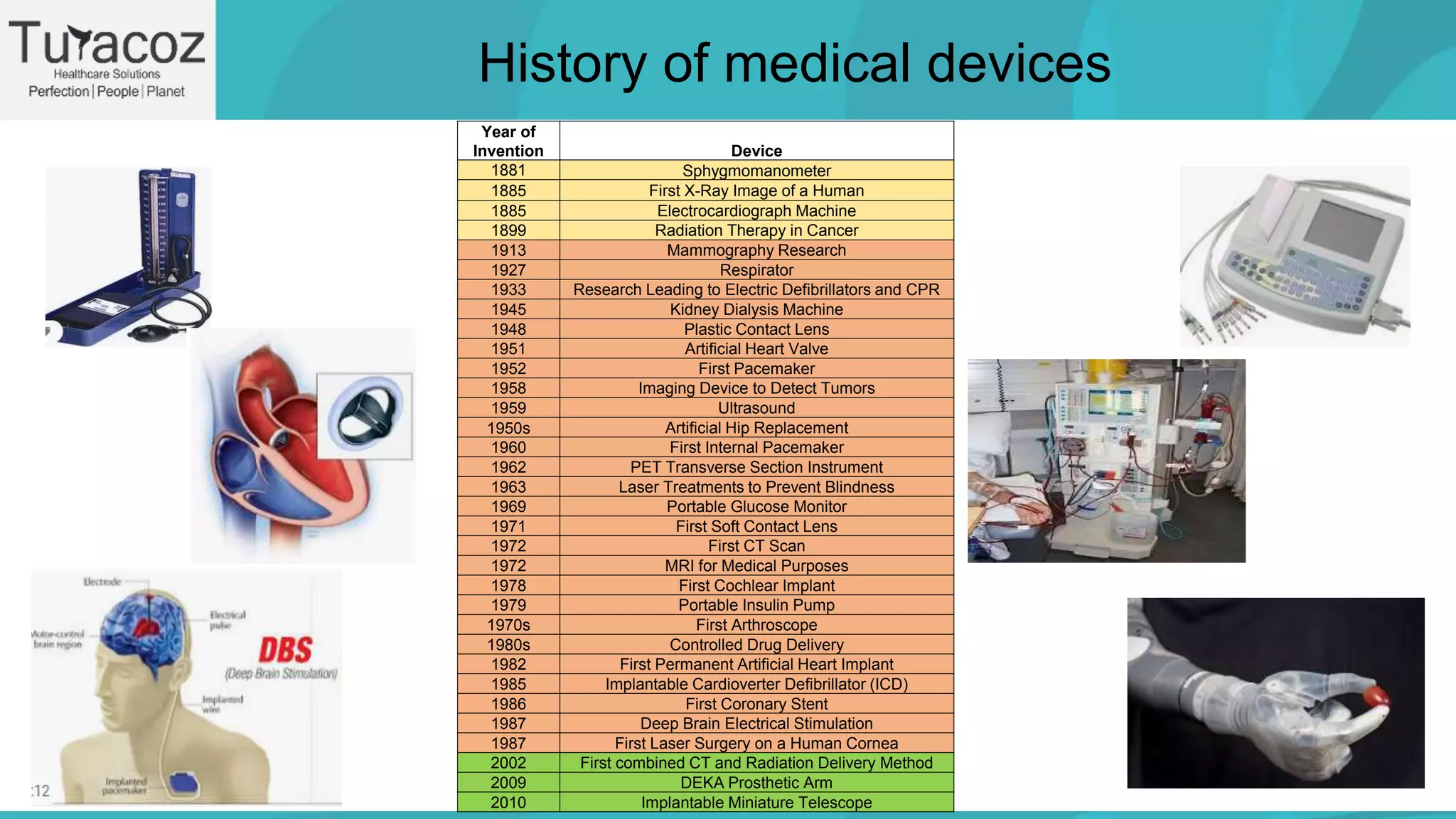
It defines medical devices as instruments used for medical purposes like diagnosis and treatment. The history section outlines important medical device innovations from the late 19th century to present. Regulations for medical devices are described, including organizations that regulate quality, safety and efficacy. Medical devices are classified based on their risks. The document discusses the drug development process, therapeutic and diagnostic uses of devices, risks, and concludes that benefits generally outweigh risks when proper risk management is followed.