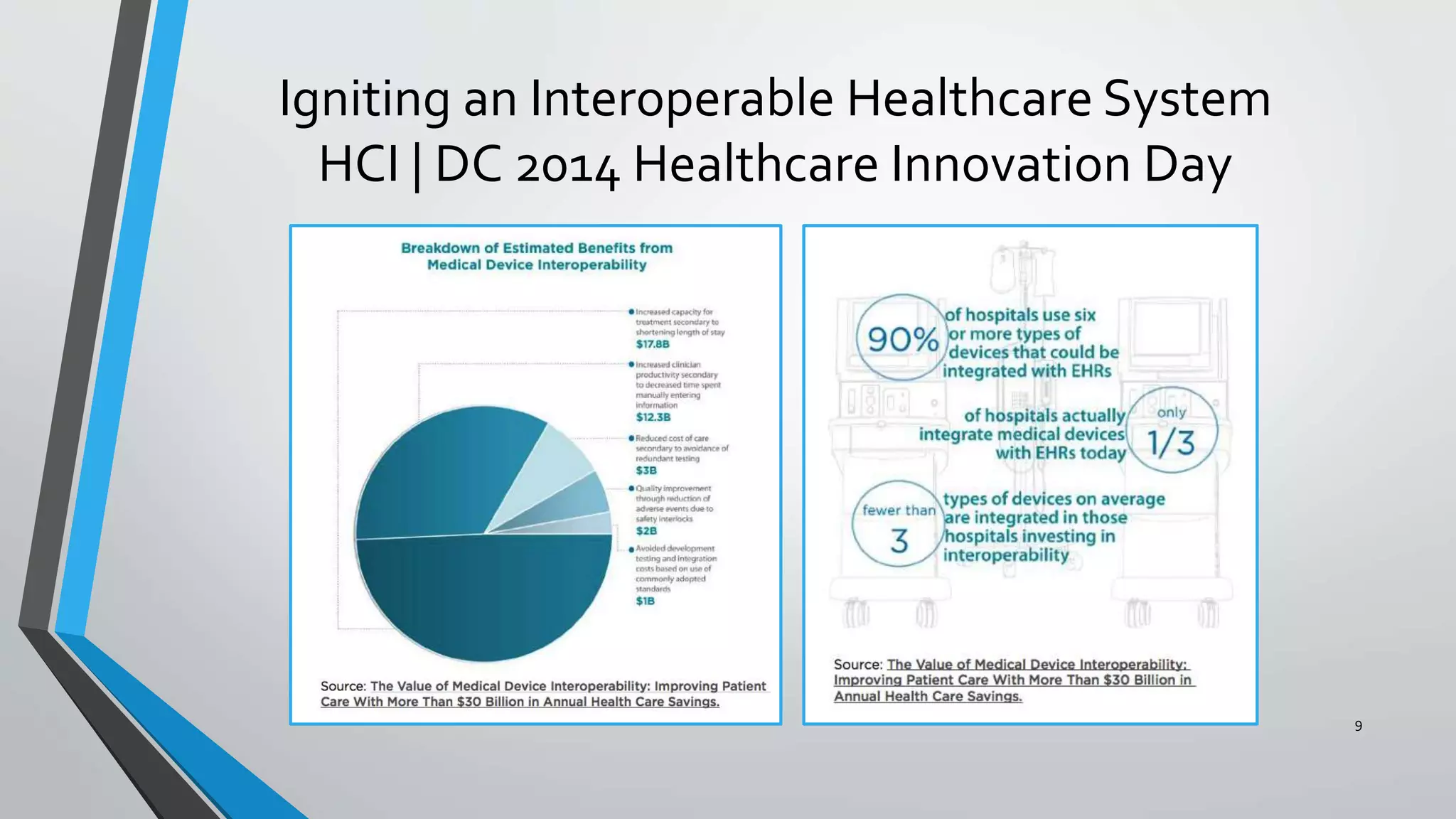
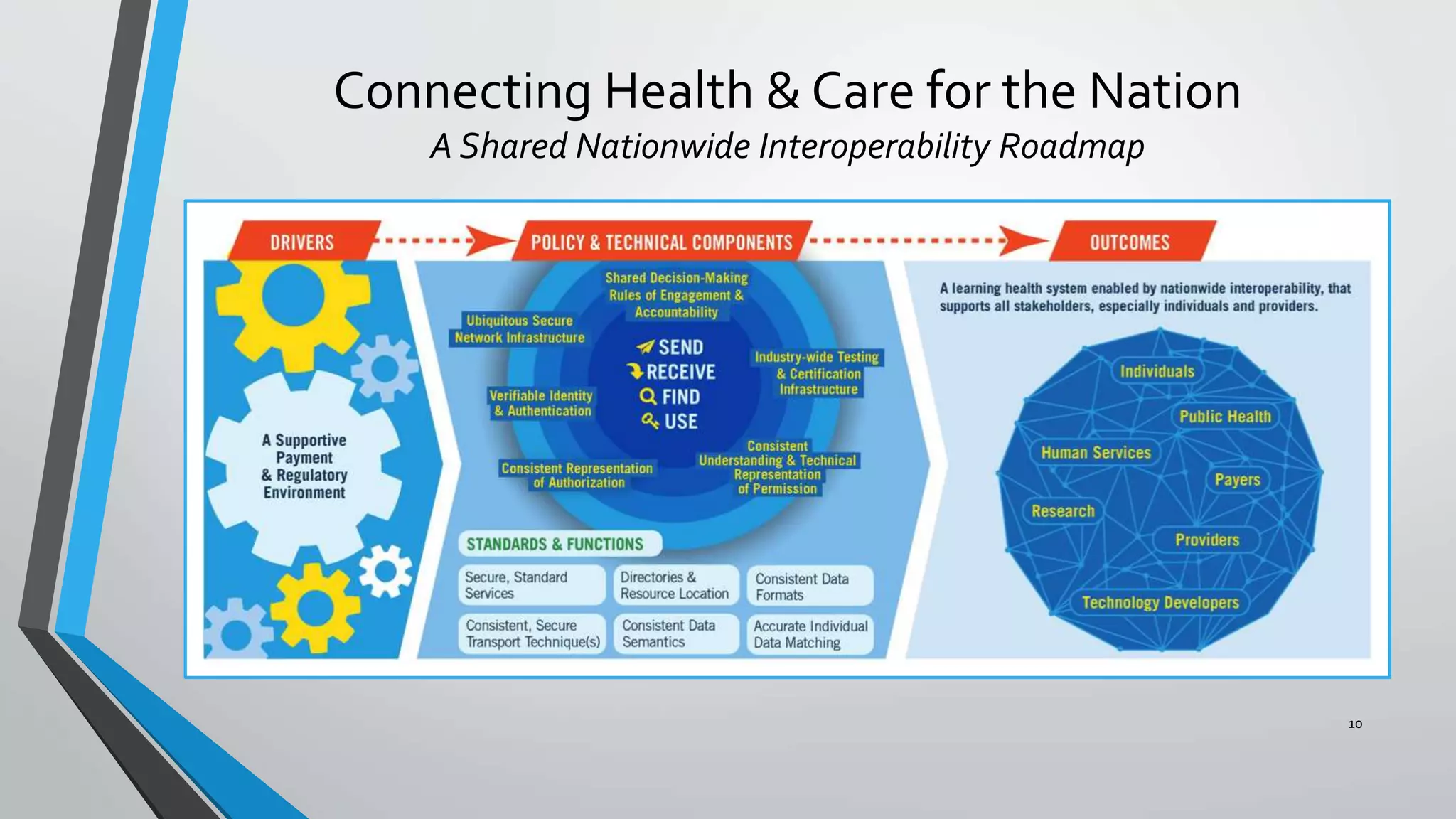
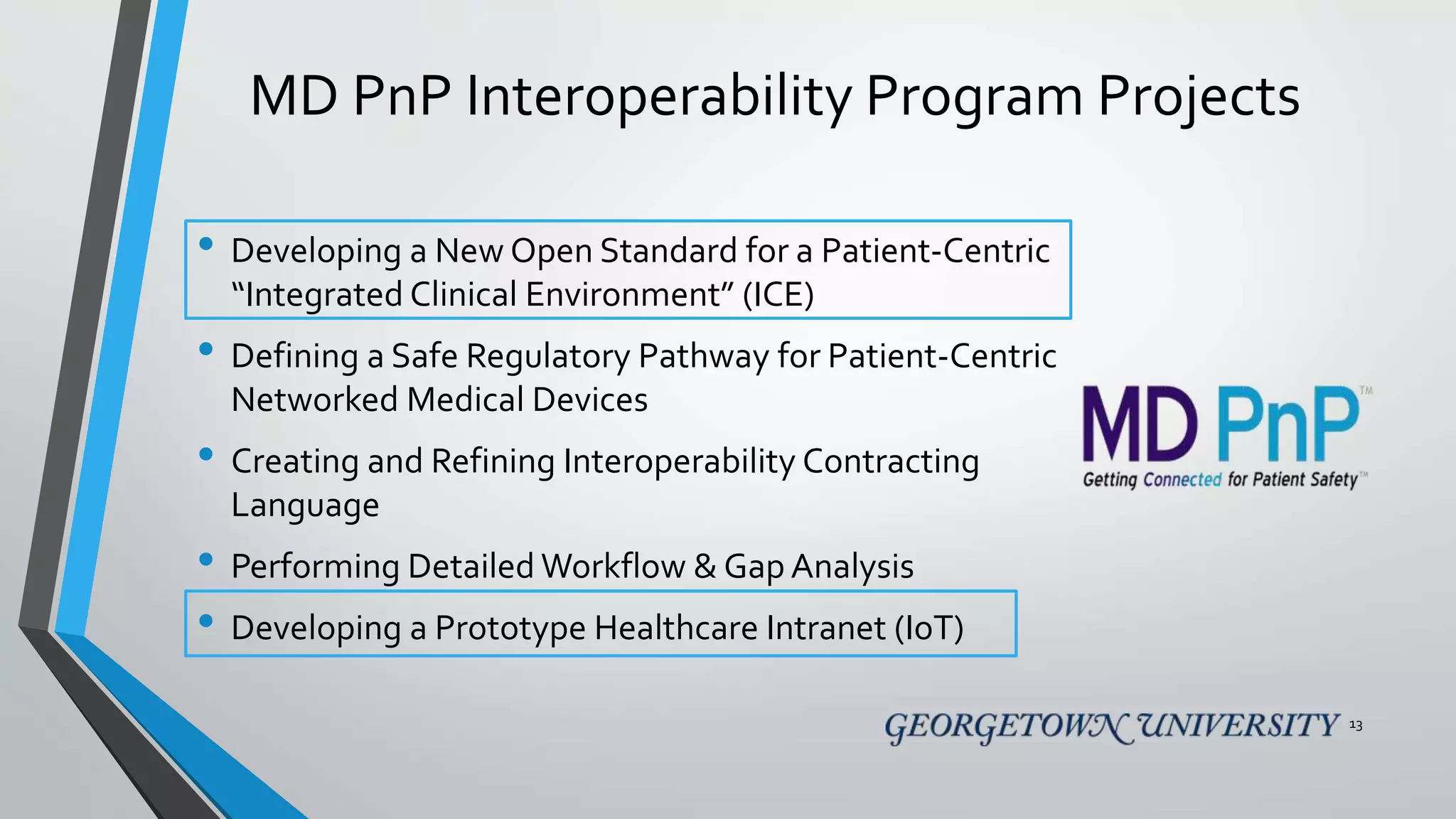
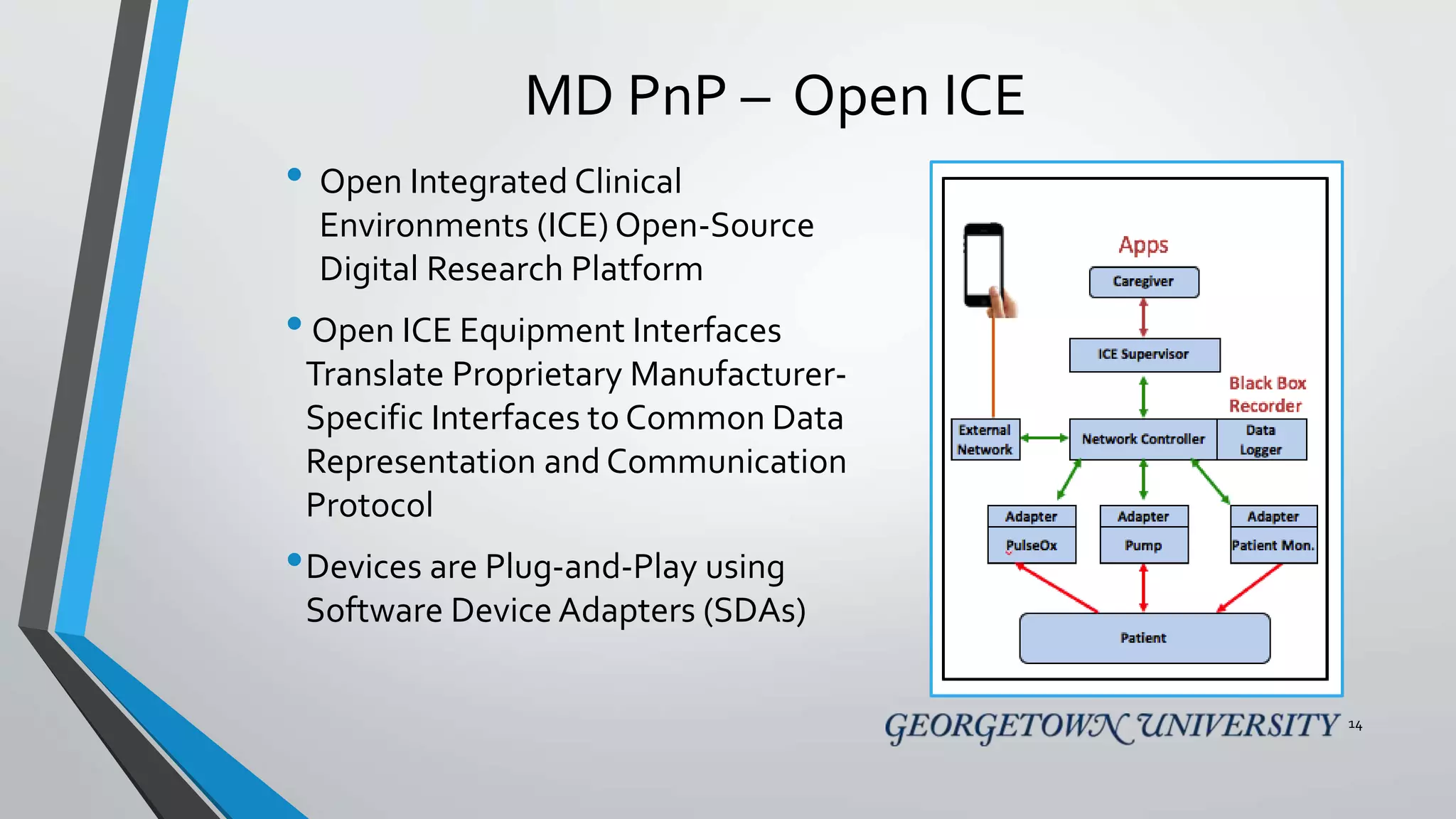
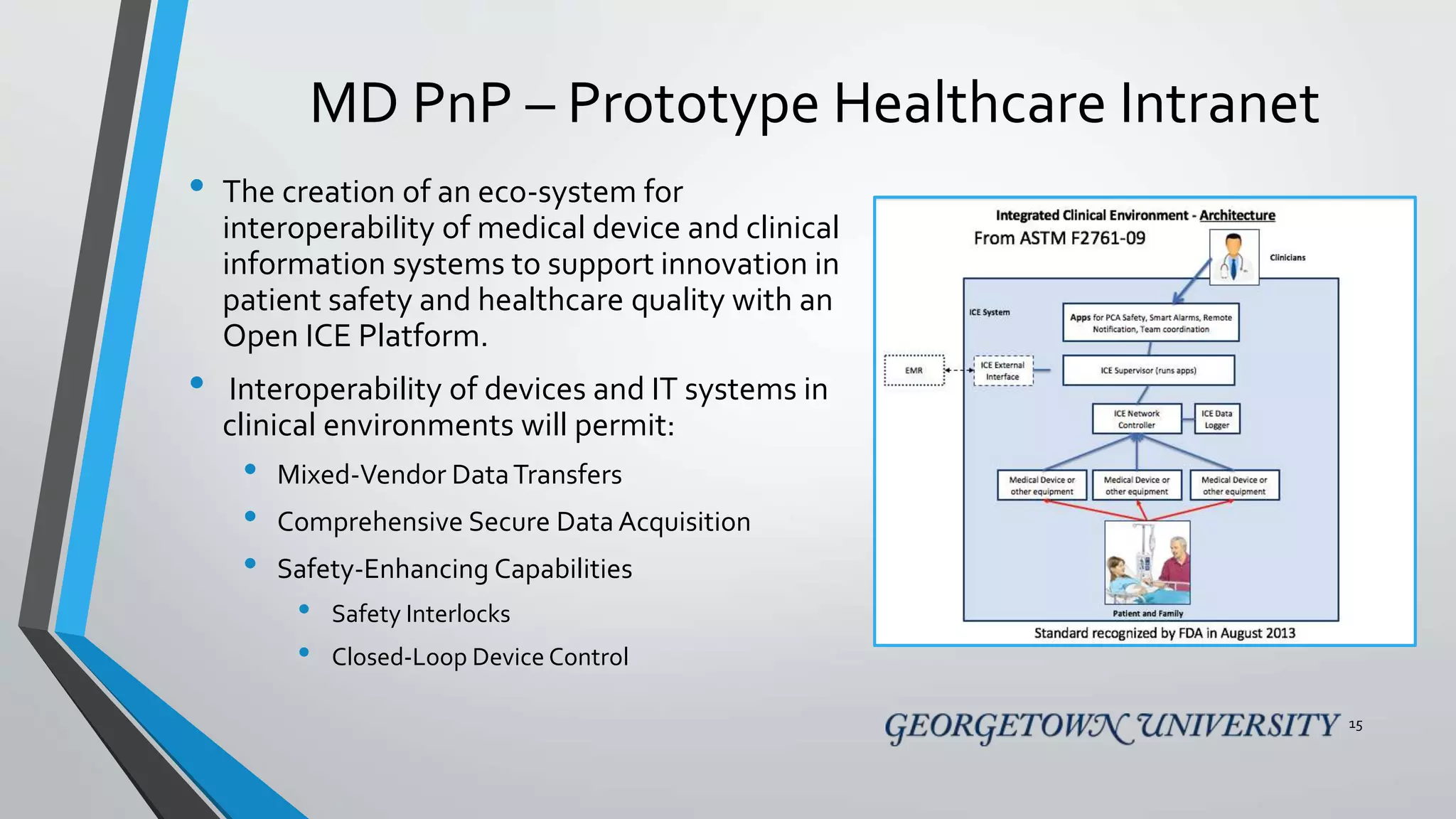
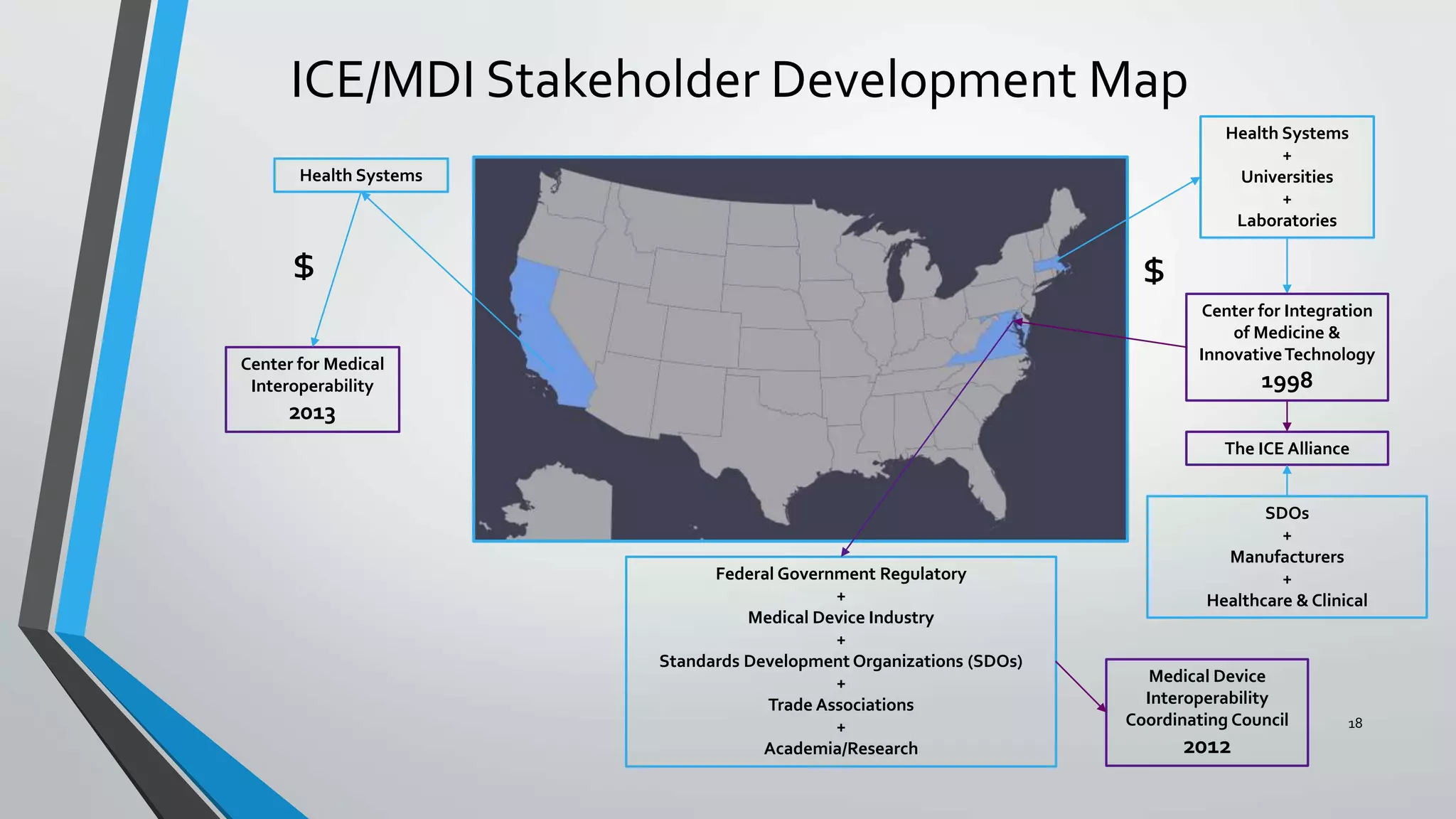
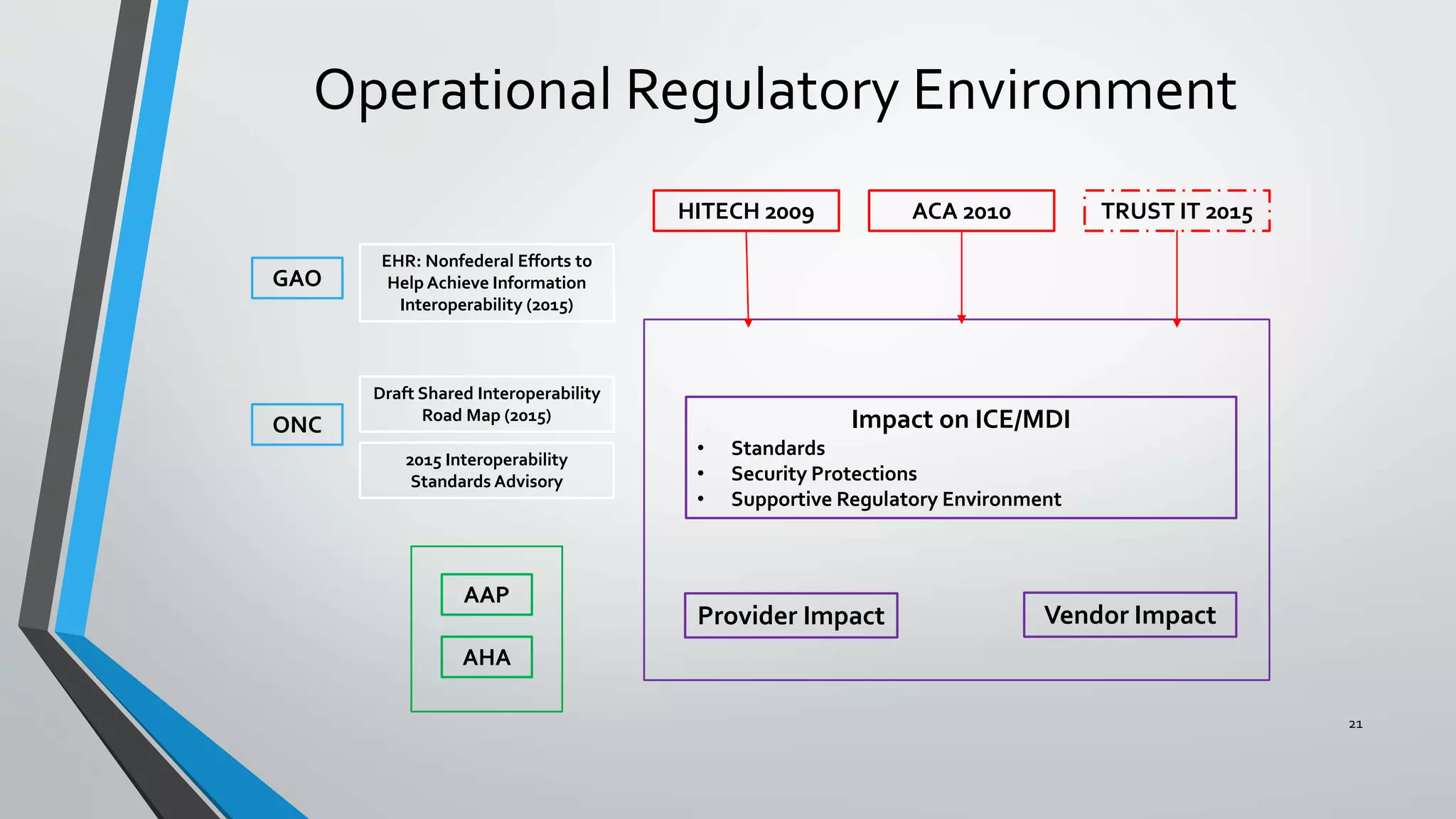
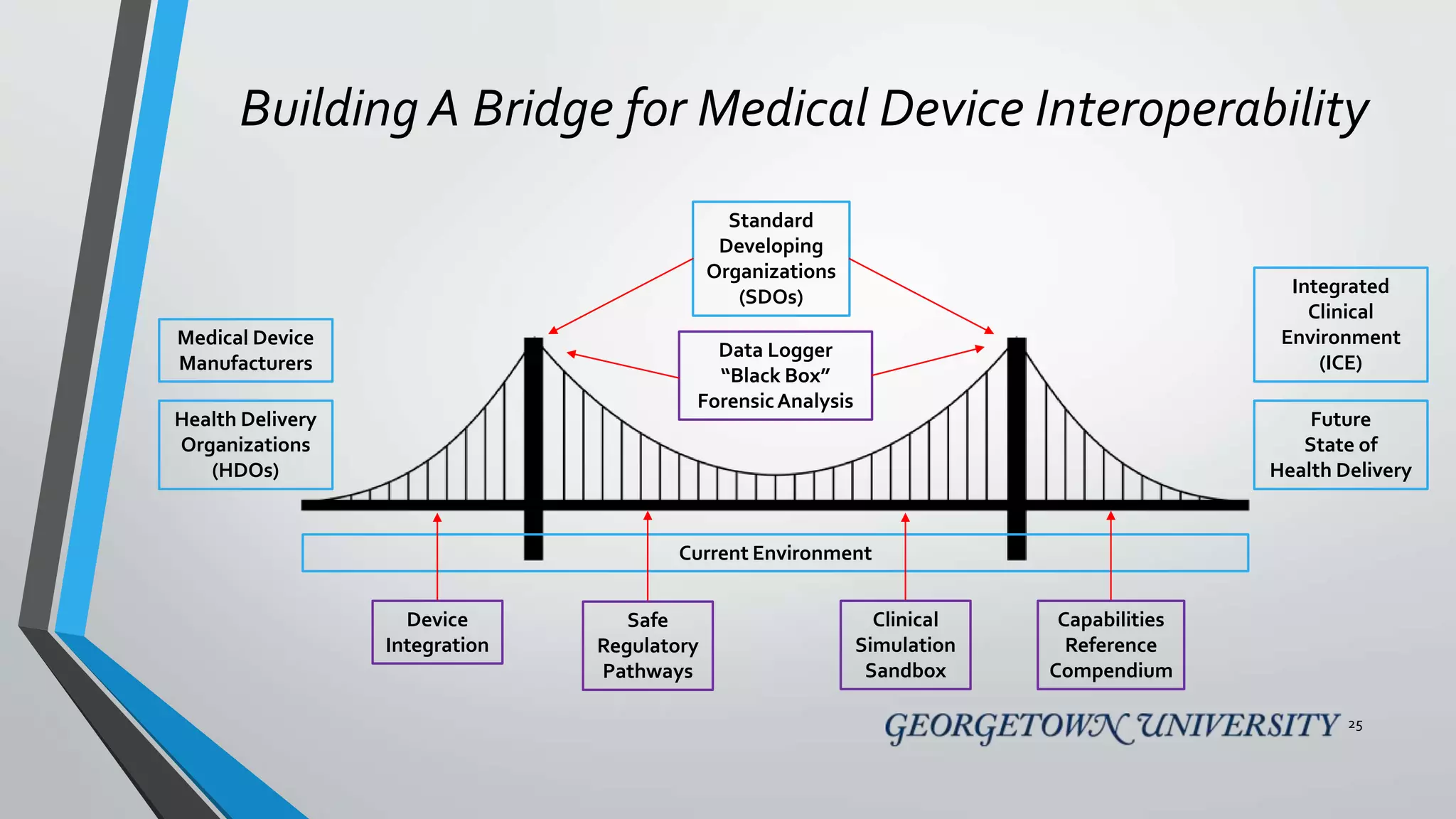
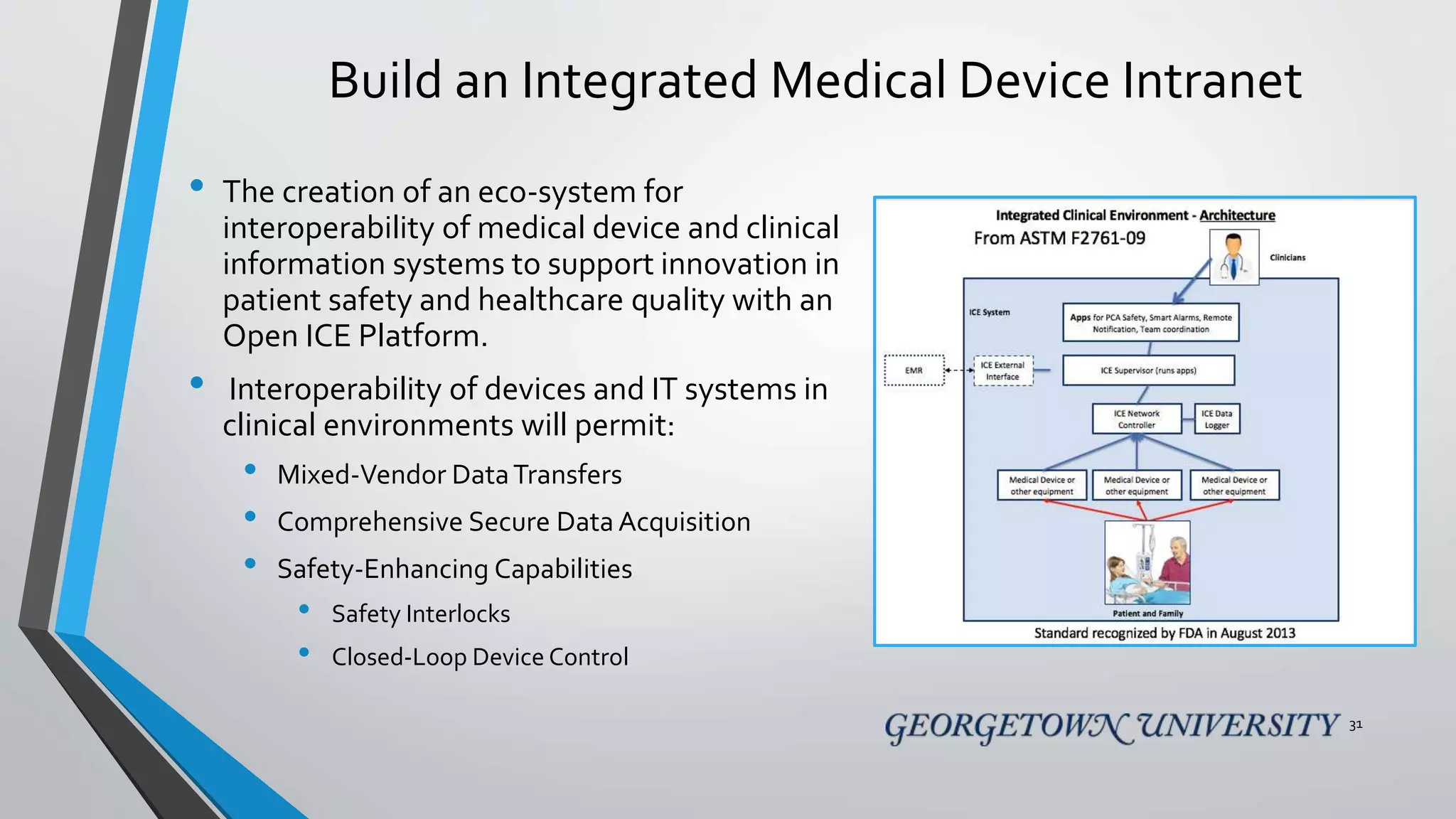
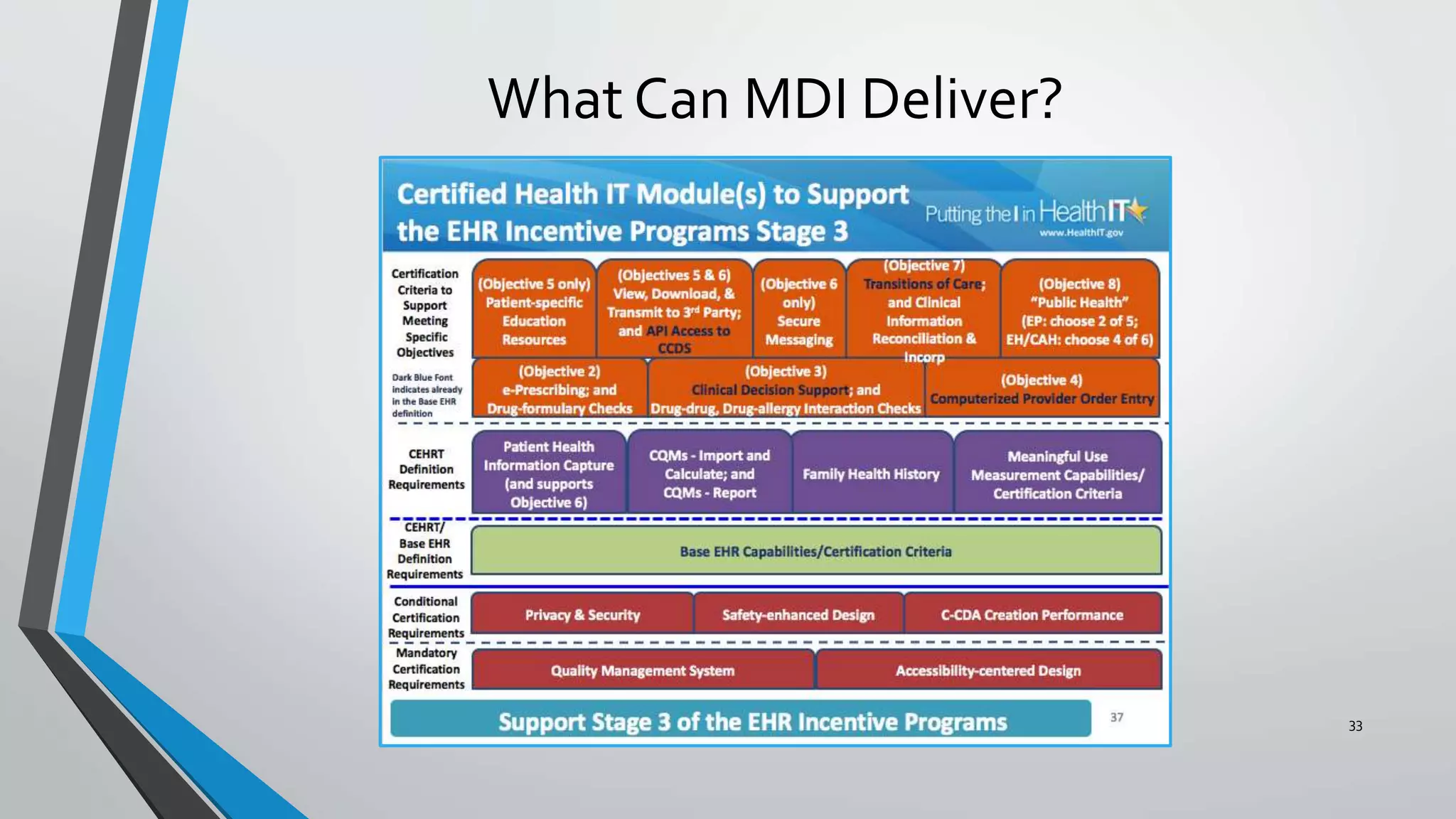
The document discusses the integration of medical device interoperability (MDI) and integrated clinical environments (ICE), emphasizing their importance in improving patient safety, healthcare delivery efficiency, and managing regulatory challenges. Key stakeholders include patients, healthcare organizations, medical device manufacturers, and regulatory agencies, all contributing to creating a robust interoperable healthcare system. The document outlines various ICE/MDI initiatives, challenges, and strategies for promoting open standards and safe regulatory pathways for advanced interoperability.