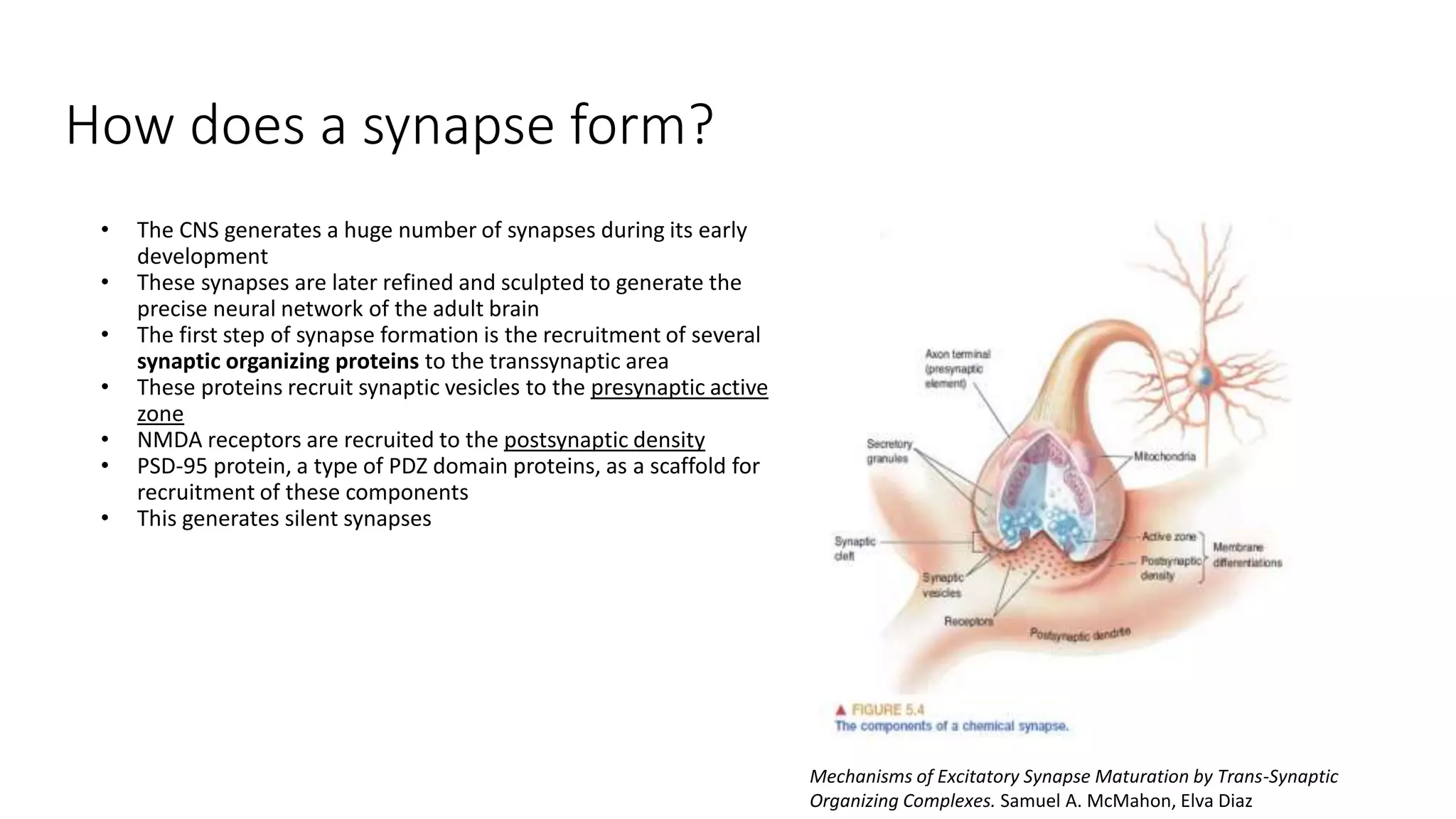
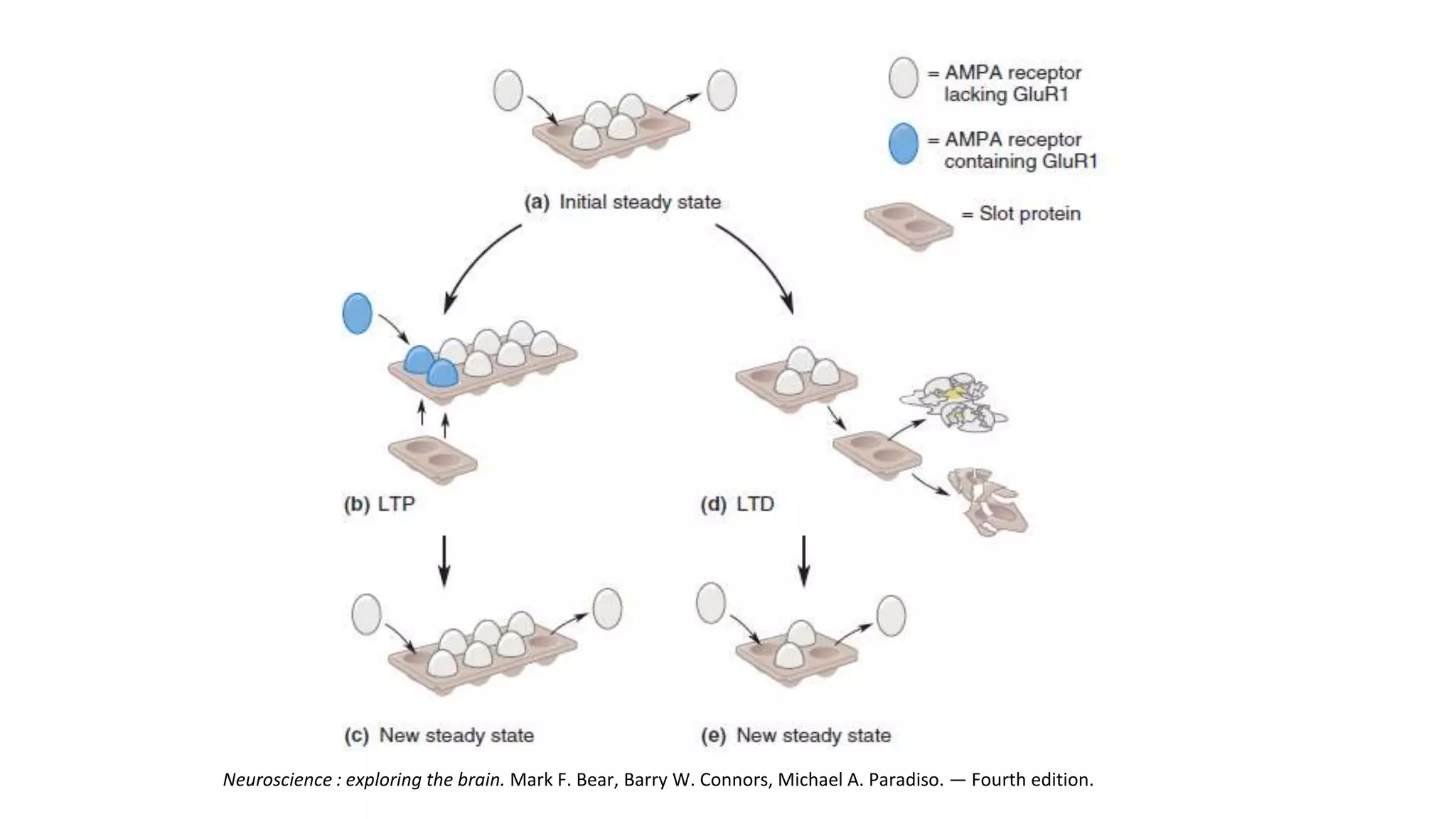
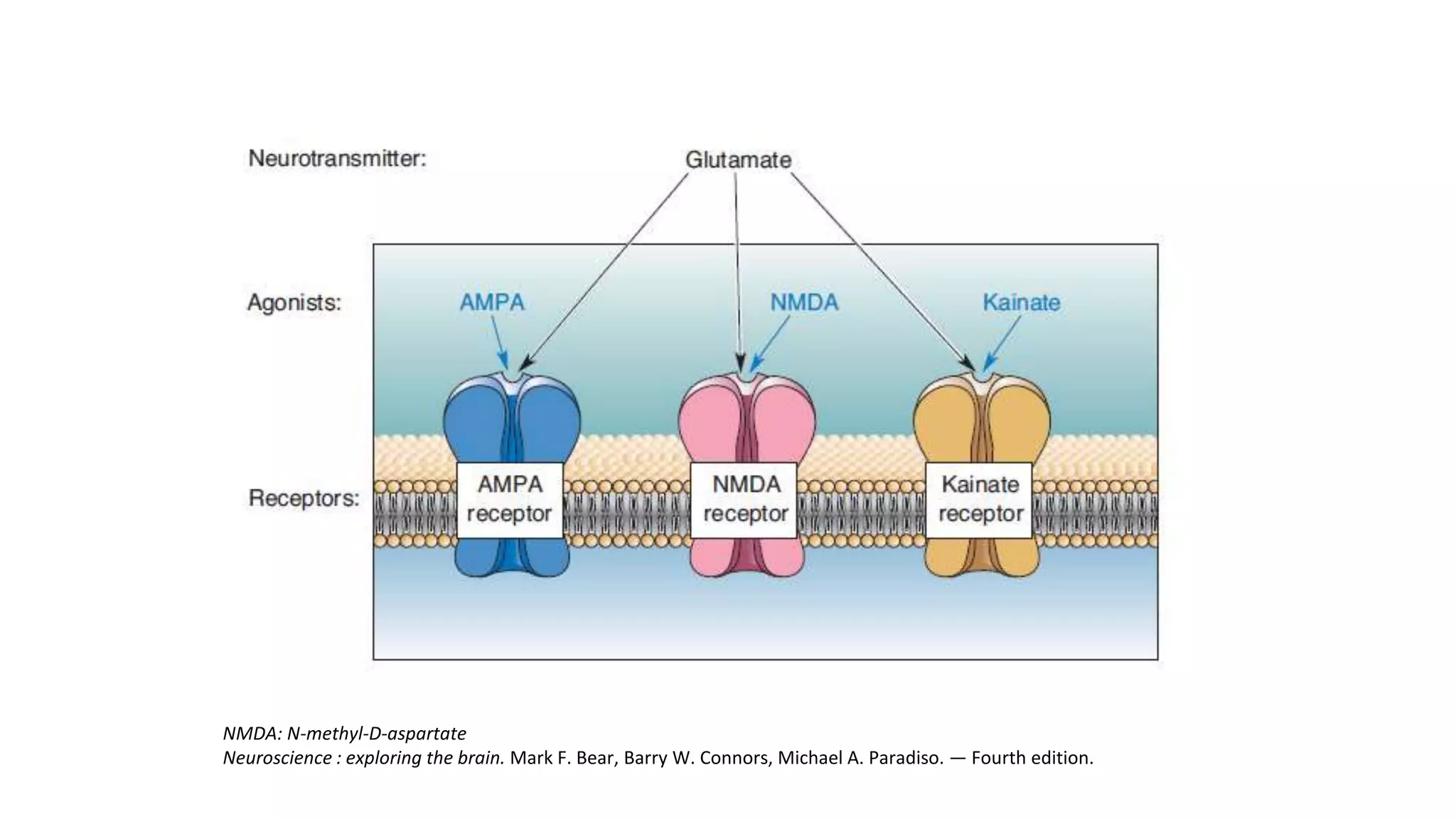
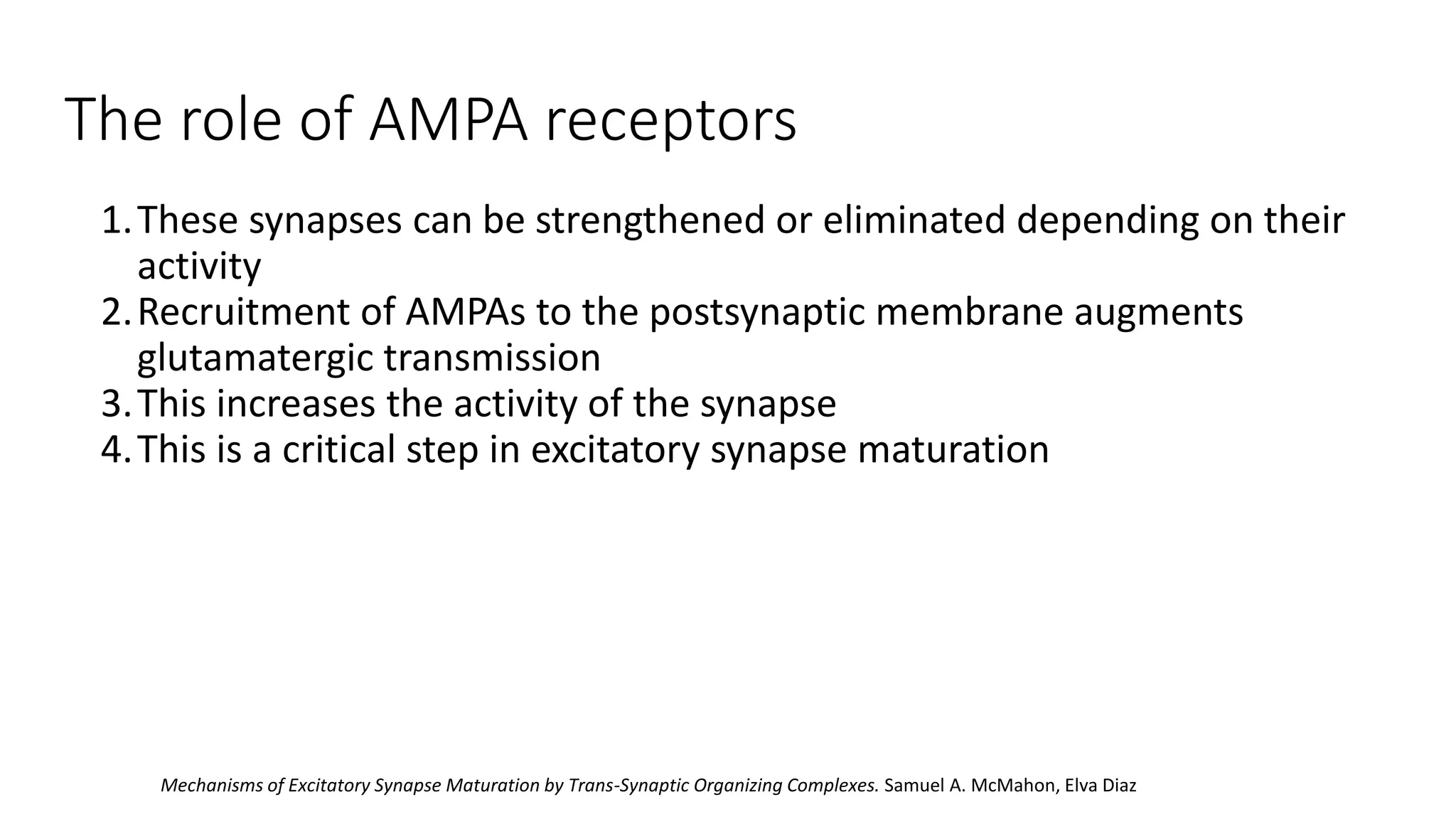
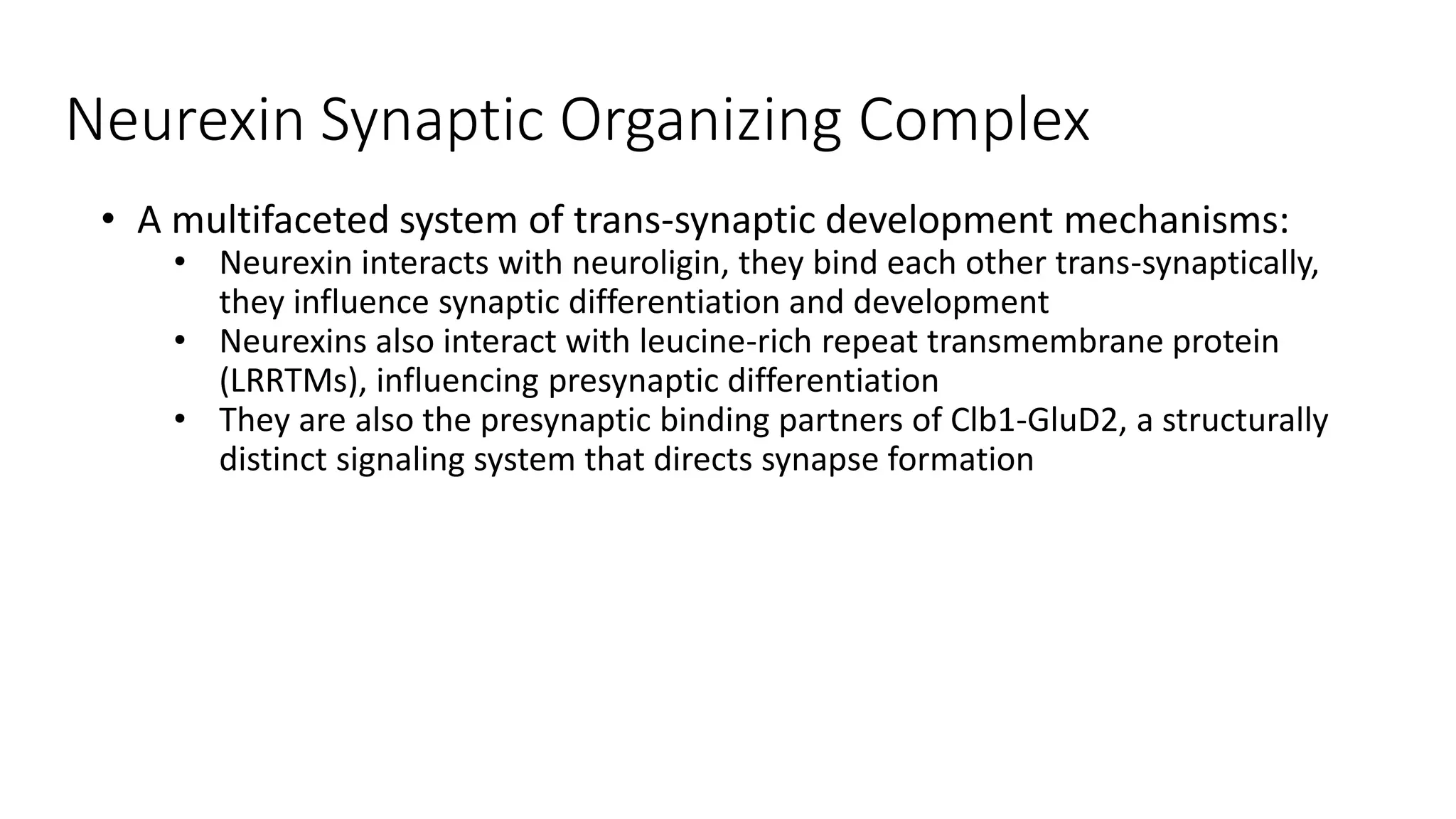
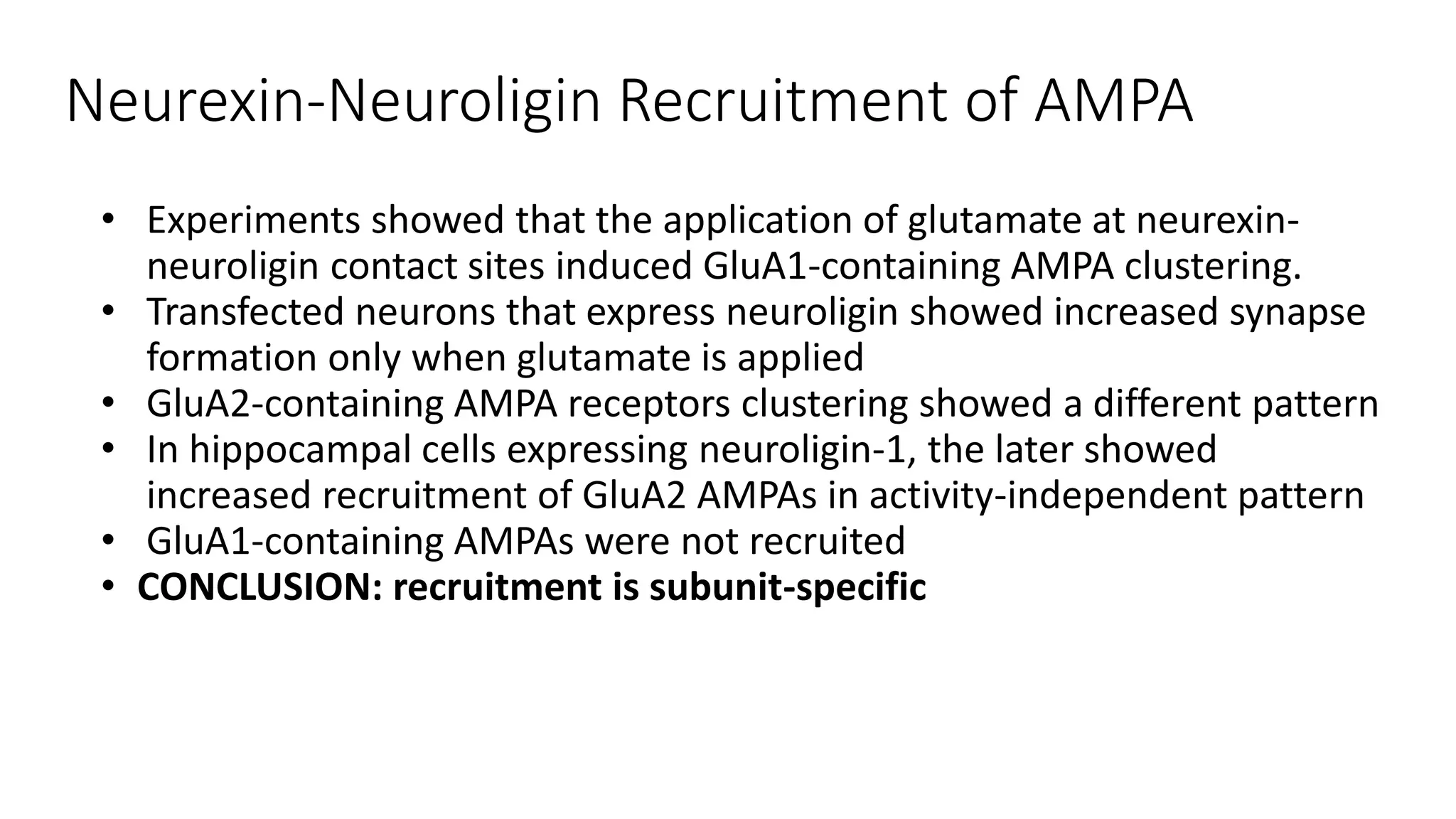
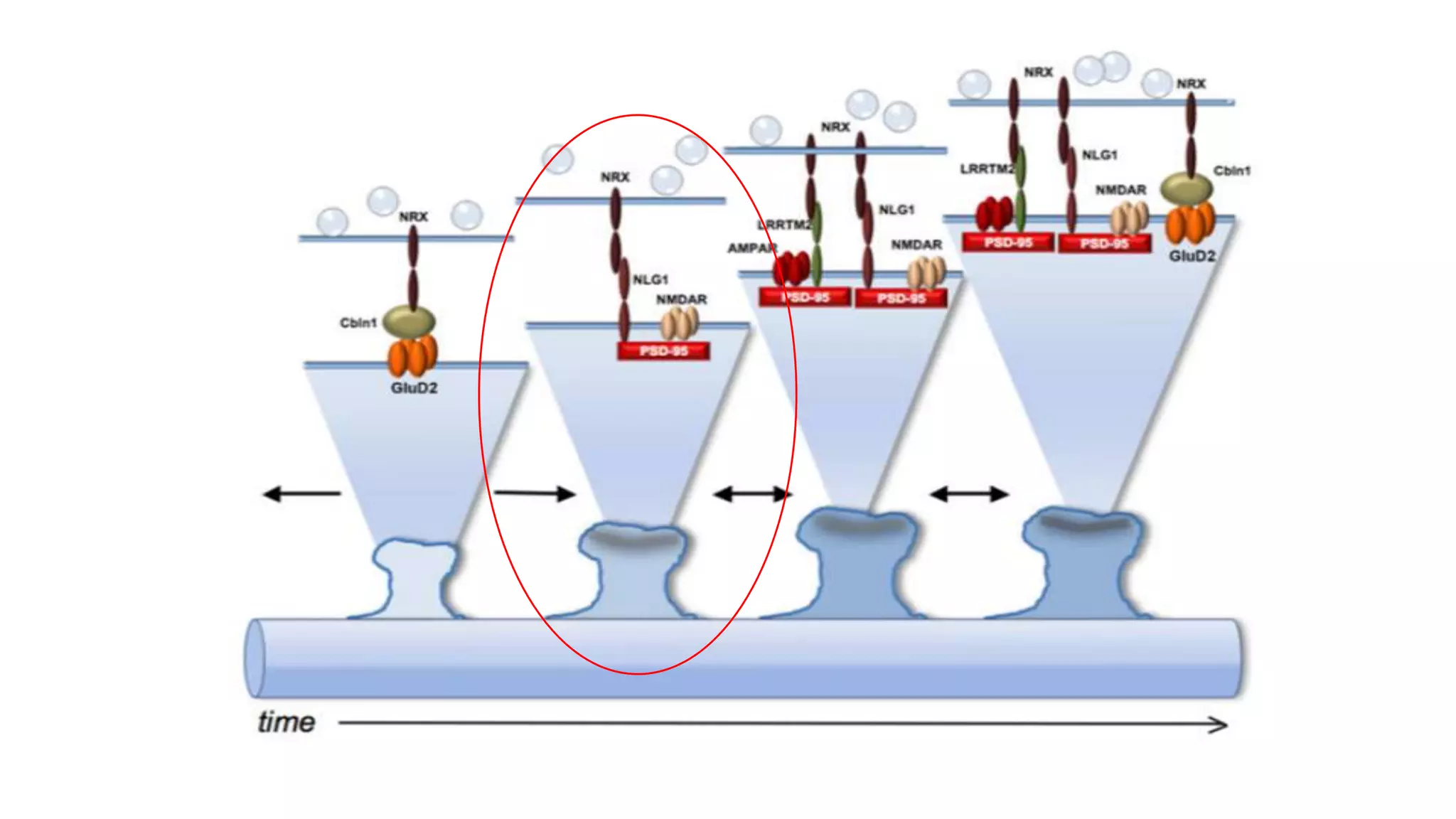
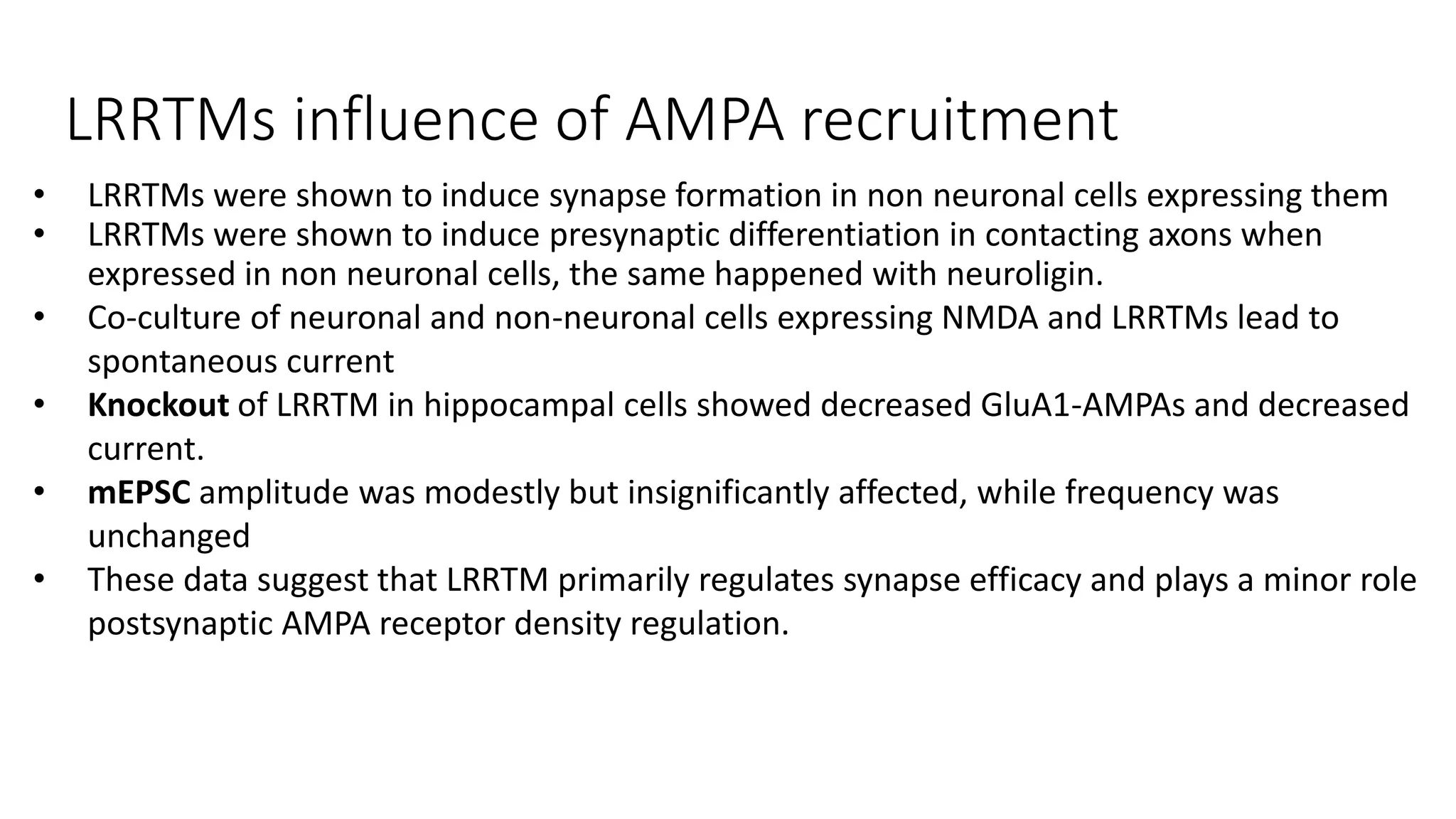
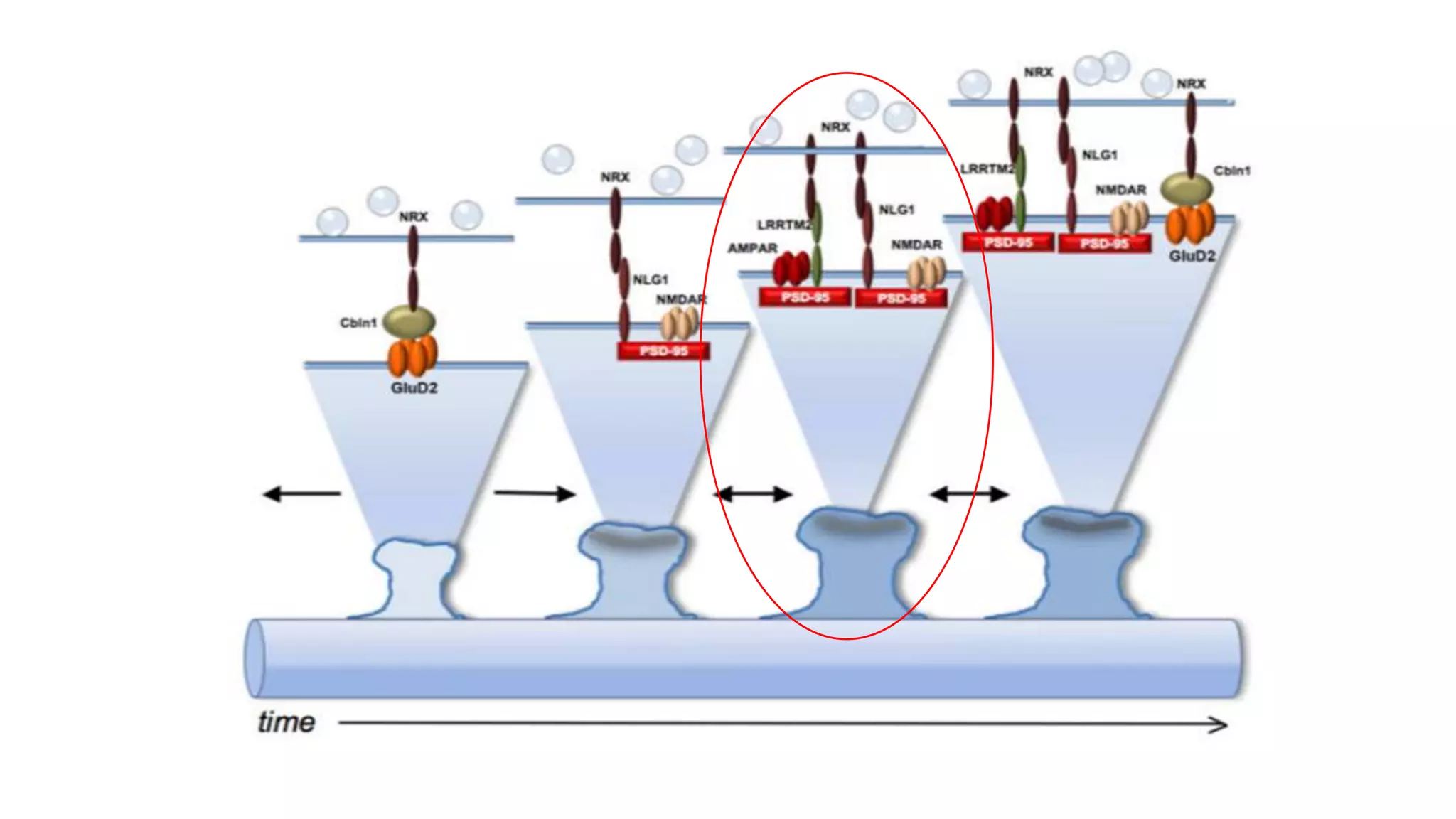
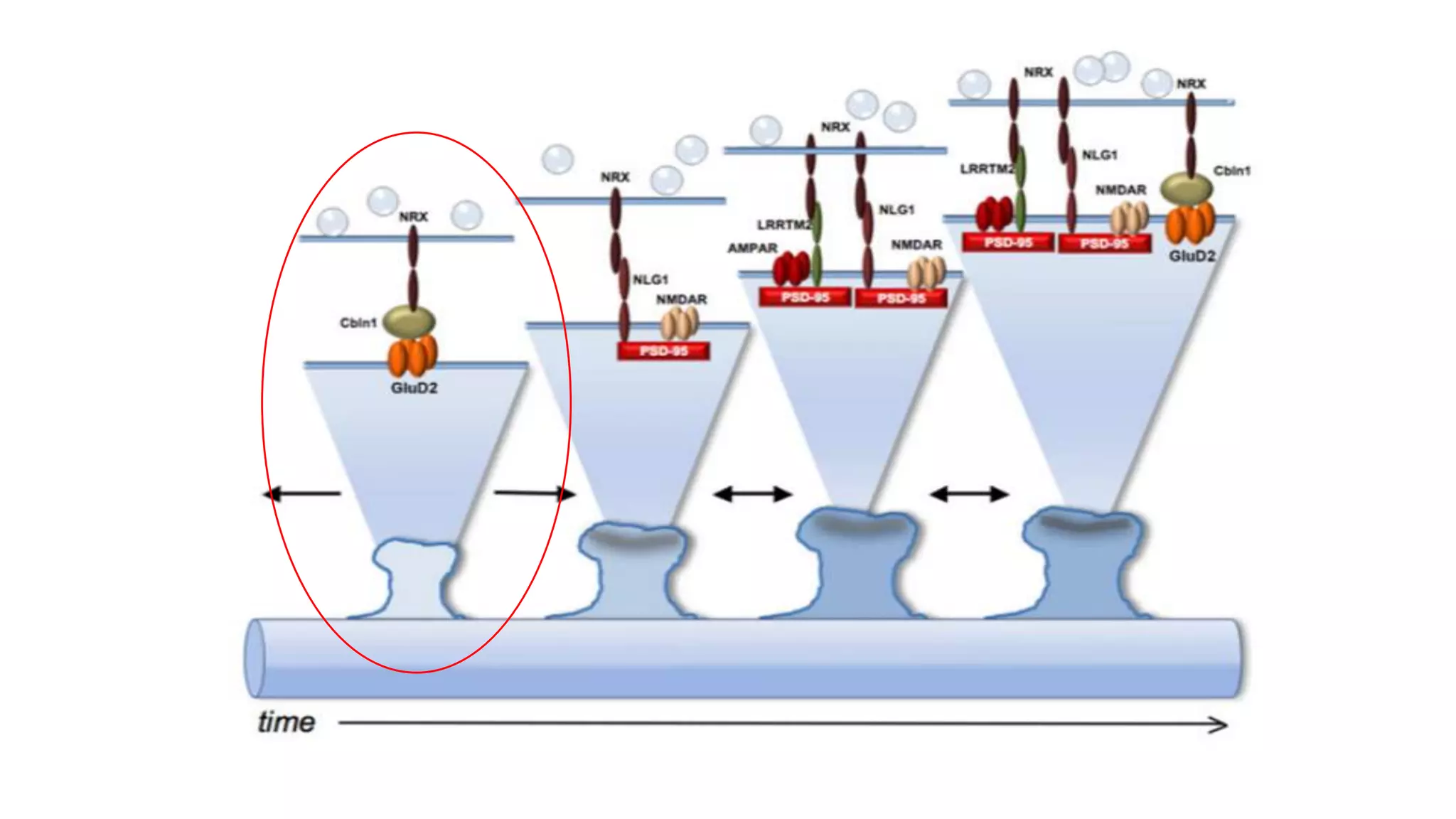
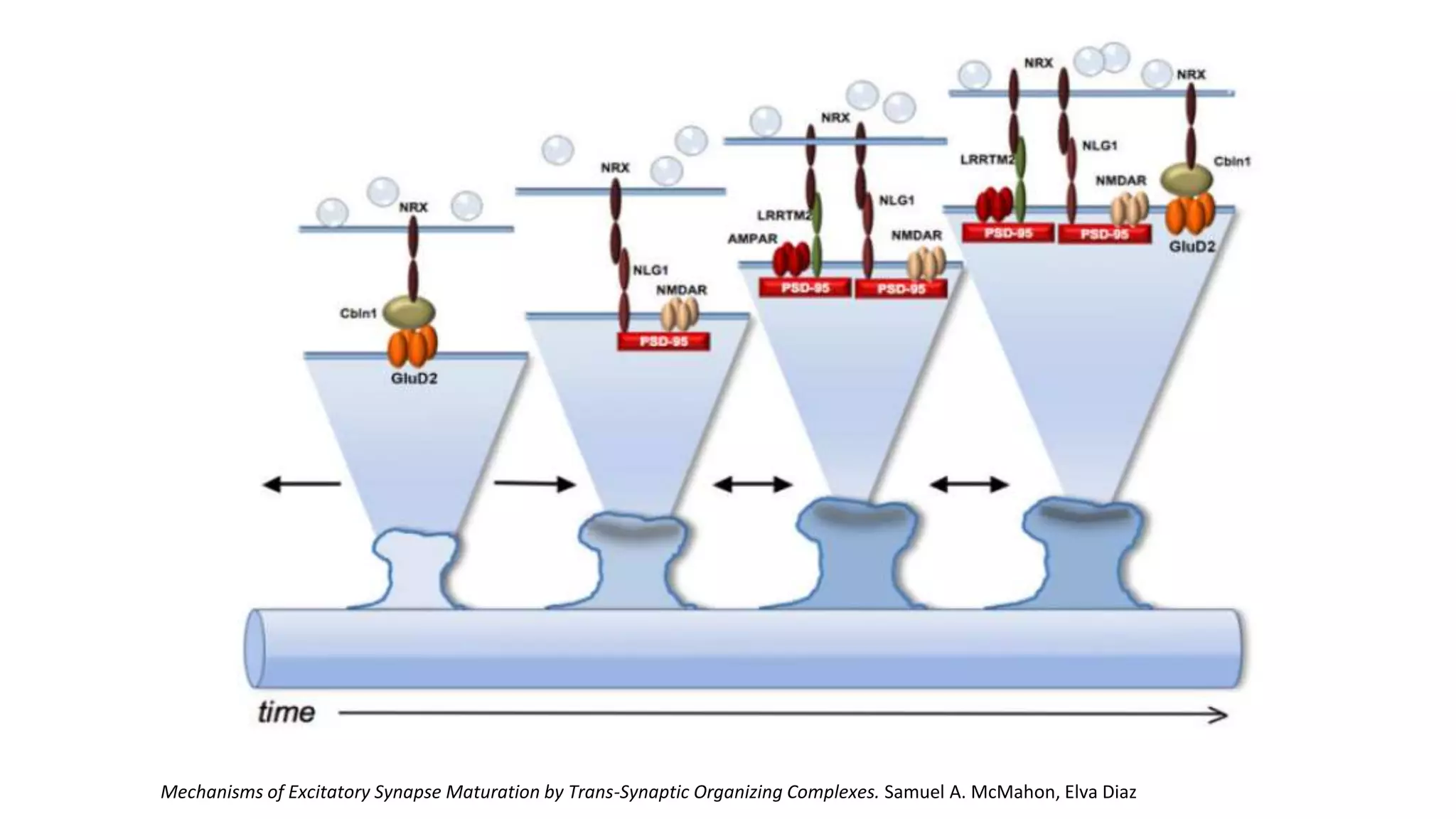
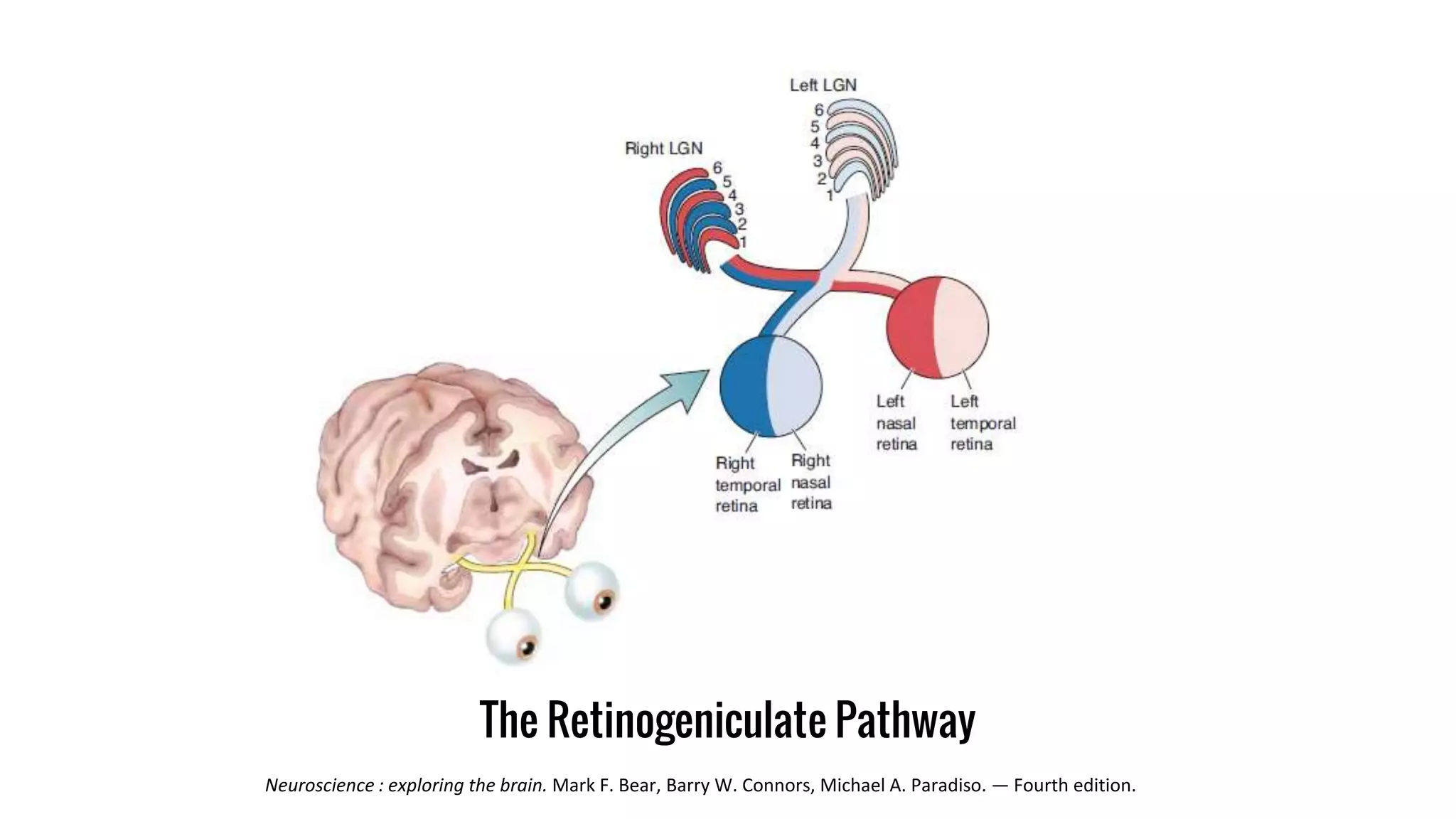
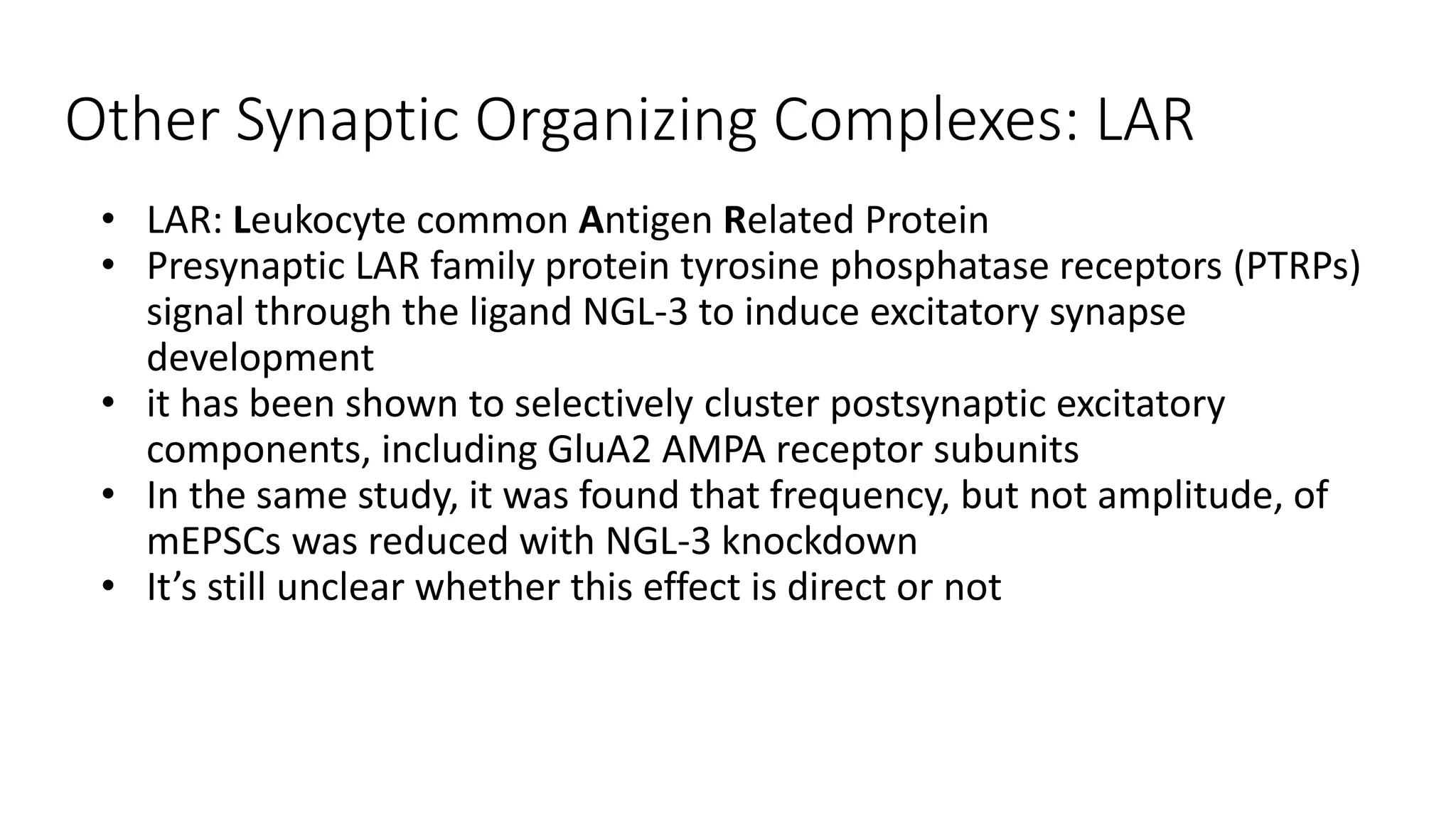
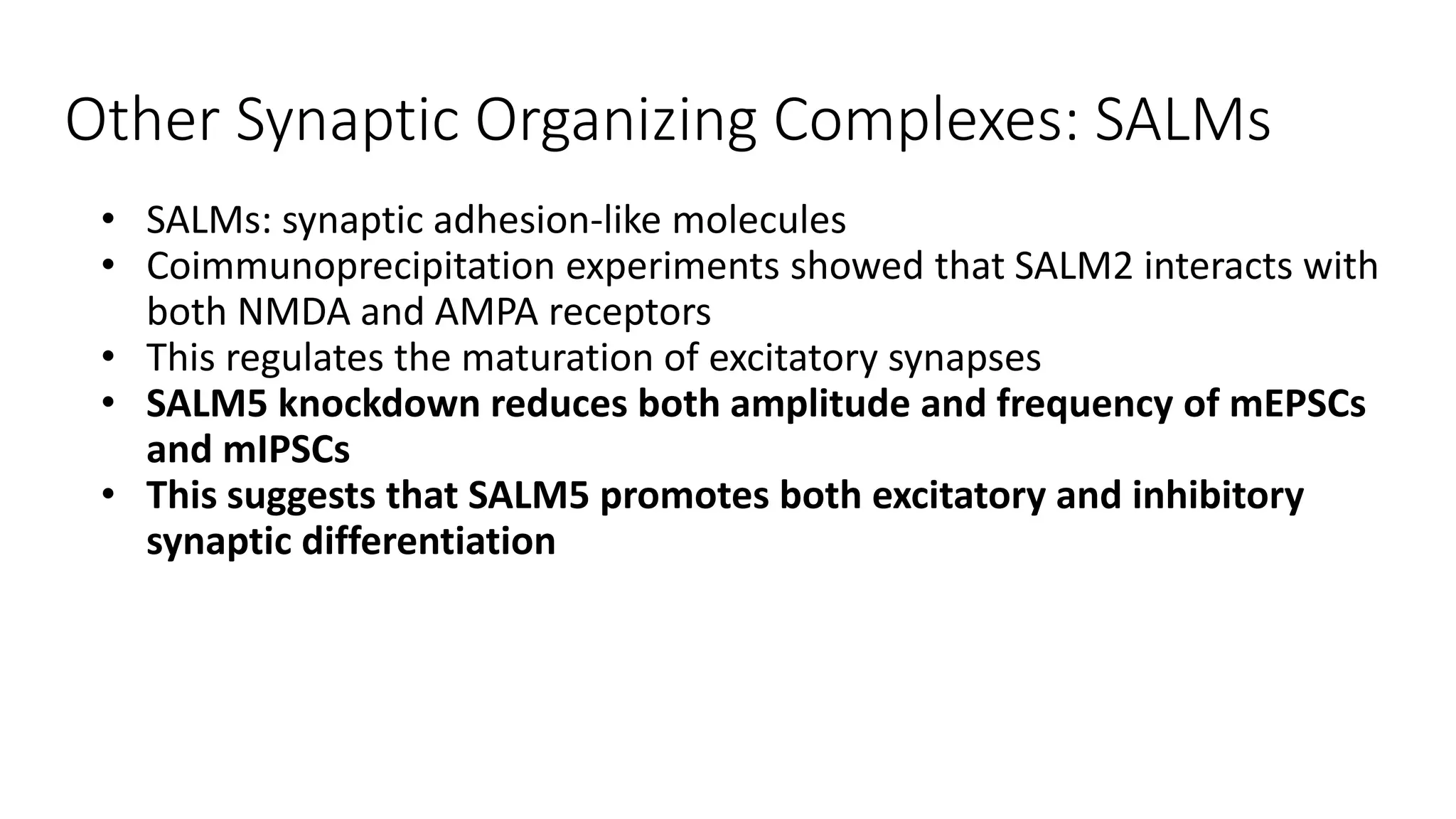
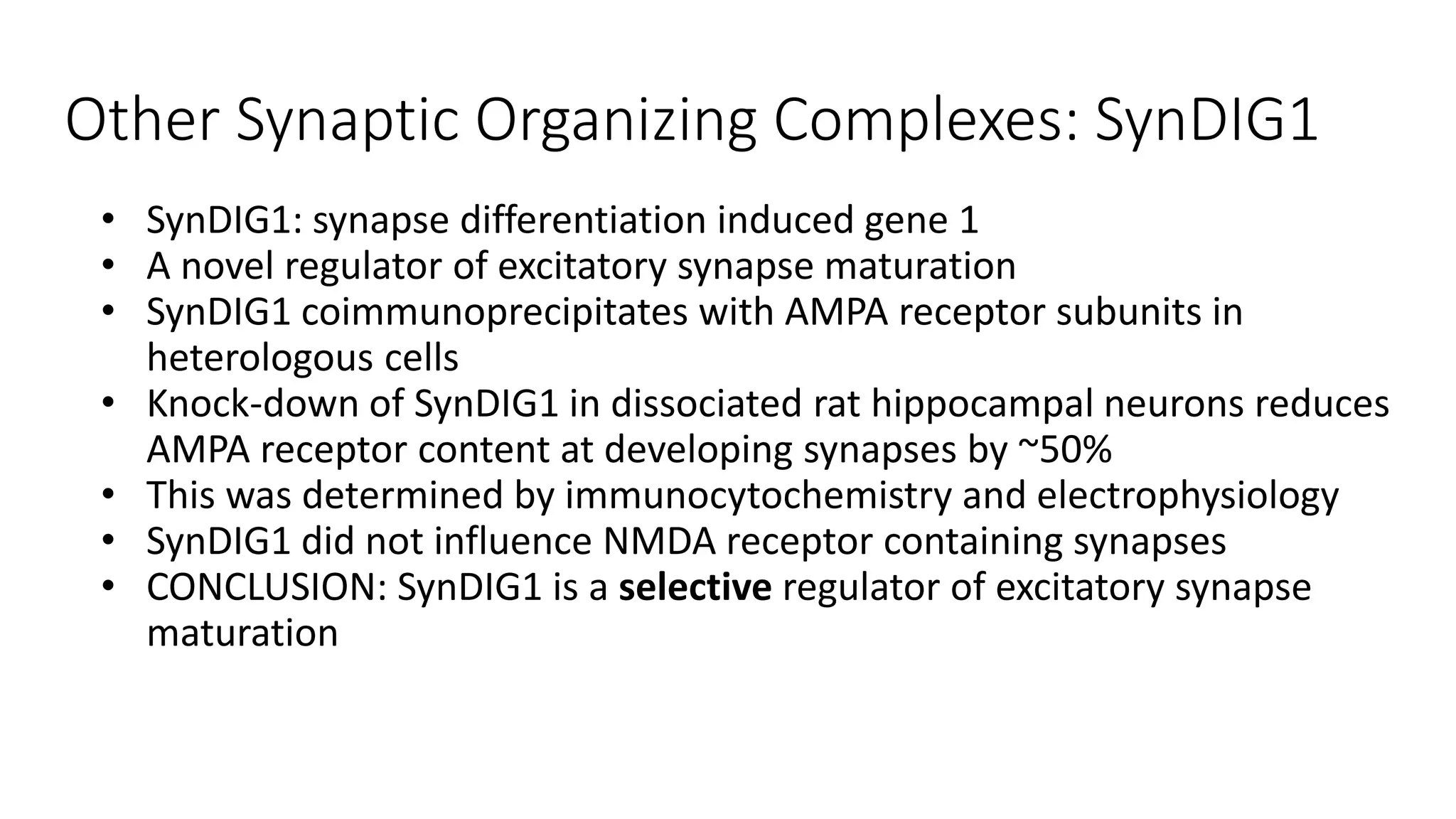
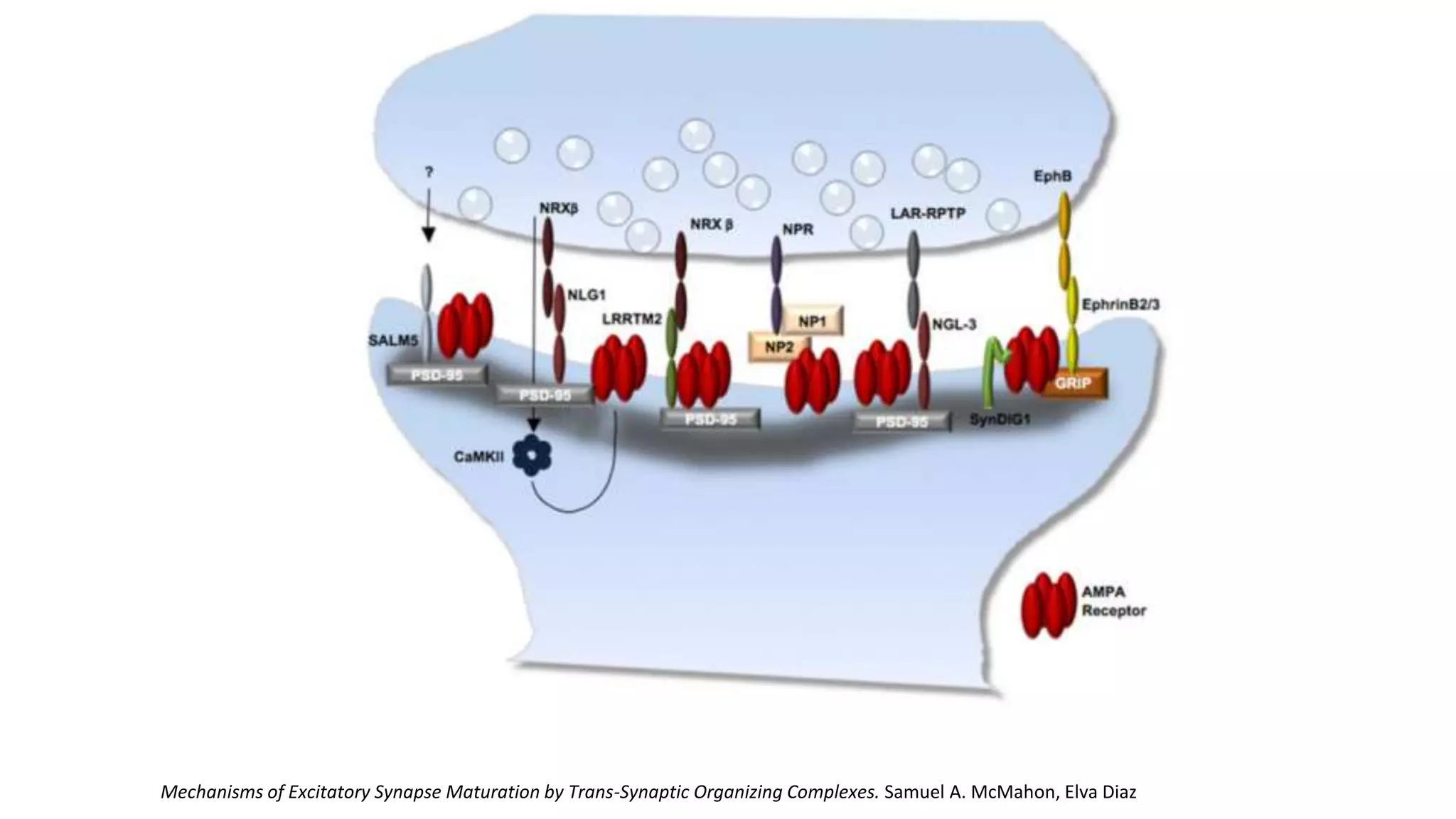
This document discusses the mechanisms of excitatory synapse maturation facilitated by trans-synaptic organizing complexes, detailing the roles of various proteins such as neurexins, AMPA, and NMDA receptors in synaptic formation and maturation. It highlights how specific proteins influence synapse efficacy and receptor recruitment, as well as the interactions between synaptic organizing proteins and their impact on neural circuitry. The document also explores the genetic and molecular pathways involved in synaptic plasticity and the implications for neurodegenerative diseases.