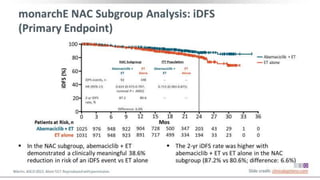
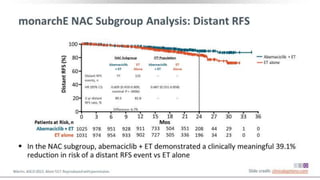
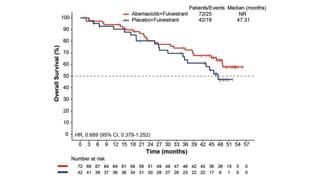
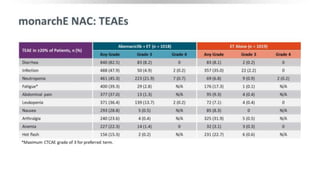
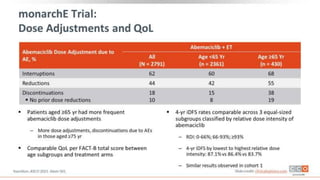
The MONARCH trial investigated the efficacy of adjuvant abemaciclib plus endocrine therapy versus endocrine therapy alone in high-risk, node-positive HR+/HER2- early breast cancer patients. Results showed that the addition of abemaciclib significantly improved invasive disease-free survival and reduced the risk of distant recurrence at 4 years. The study concludes that the benefits of abemaciclib are sustained beyond treatment completion, warranting further follow-up to assess overall survival improvement.






















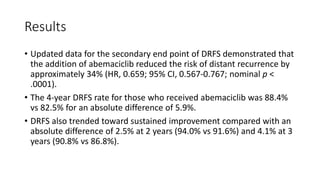

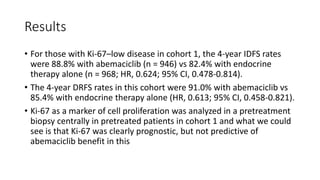
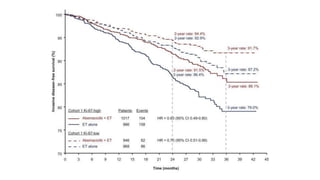
![Results
• Patients with a high Ki-67 index treated with endocrine therapy alone
had a much worse prognosis. “[For those who received endocrine
therapy alone], 25% have relapsed by 4 years, compared with 17%
with a low Ki-67]”.](https://image.slidesharecdn.com/monarcheautosaved-230824173107-4082ed3a/85/MonarchE-31-320.jpg)