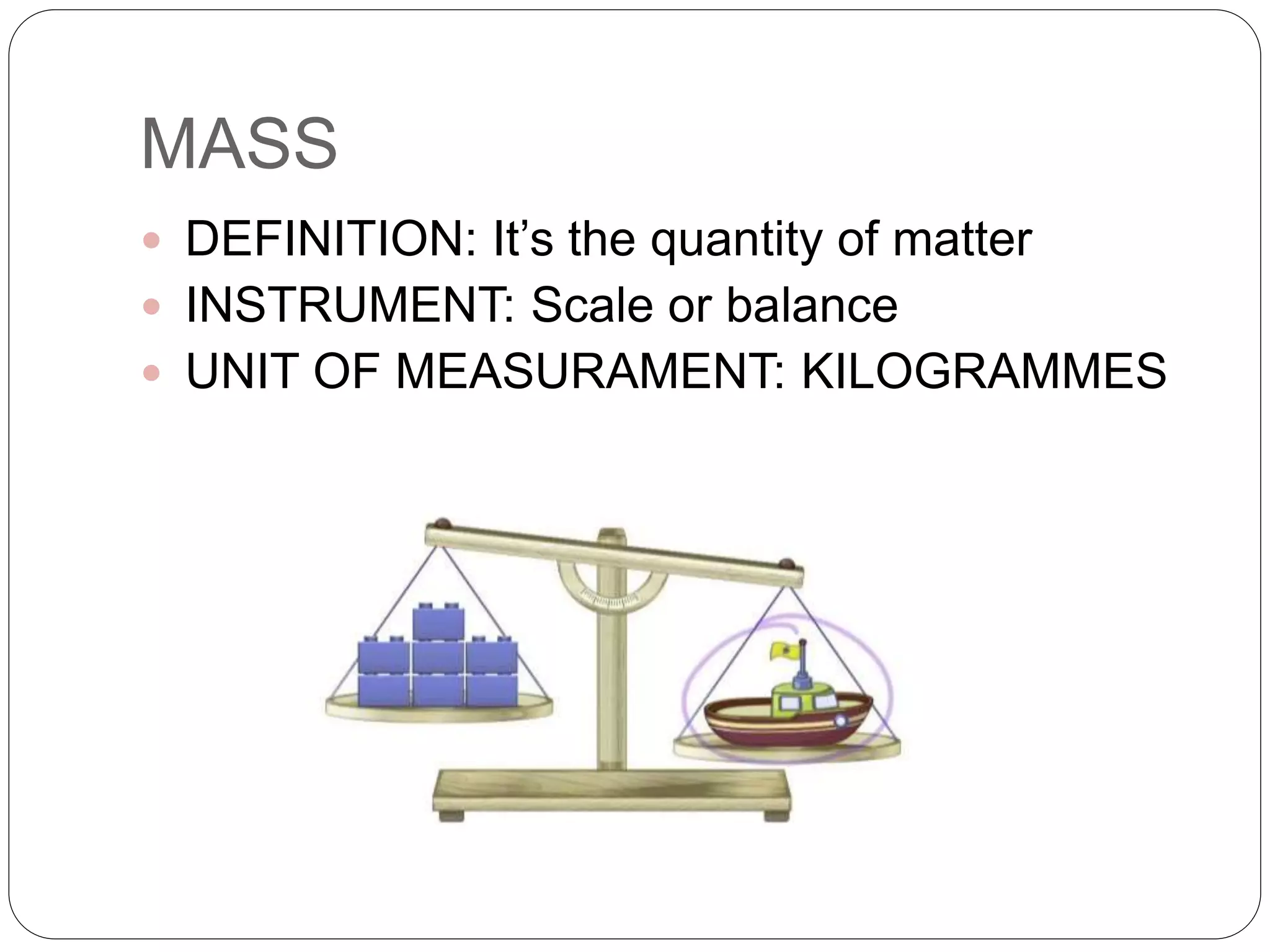
The document discusses the properties and types of matter. It defines matter as anything that takes up space. All matter has two general properties - mass and volume. Mass can be measured with a scale or balance using kilograms as the unit, while volume is the space an object occupies and is measured using liters. Matter can be classified as pure substances made of only one component or mixtures made of two or more substances that can be either homogeneous, where the components cannot be distinguished, or heterogeneous, where the components can be distinguished. Common examples of each are provided.