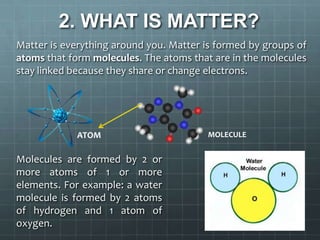
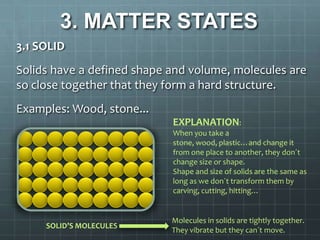
This document discusses matter, materials, and recycling. It defines matter as everything around us, formed by atoms and molecules. It describes the three states of matter - solids have a fixed shape and volume, liquids take the shape of their container, and gases fill their container. Materials are made from matter and can be natural like wood, metal, and stone, or artificial like plastic and glass. The document outlines properties of materials like strength, flexibility, and weakness. It emphasizes the importance of recycling materials to preserve the environment and lists common recycling bins for different waste types.