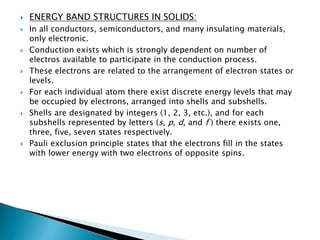
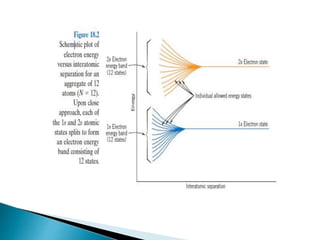
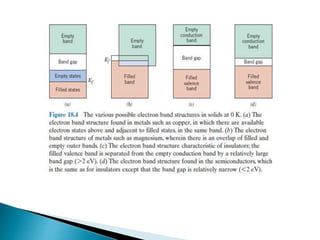
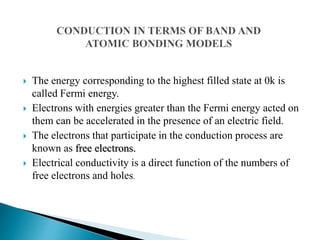
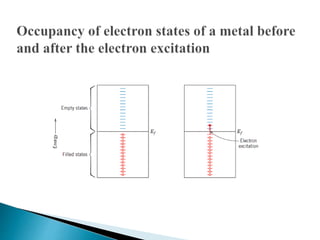
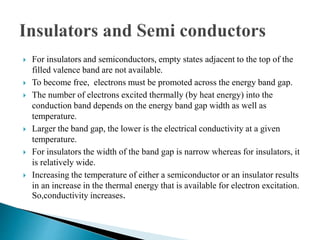
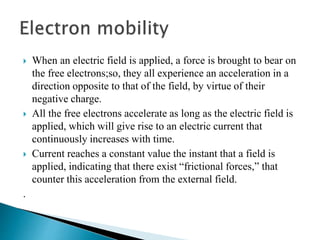
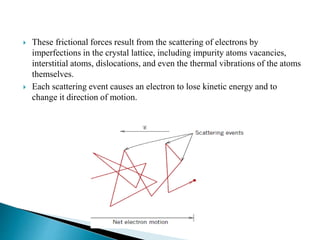
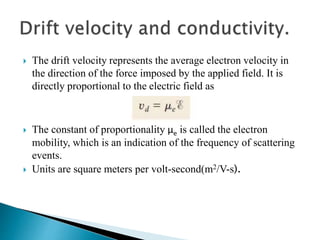
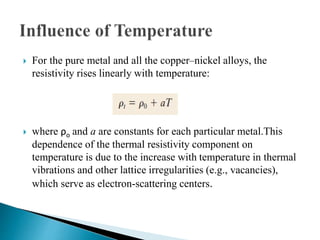
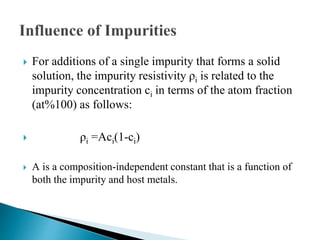
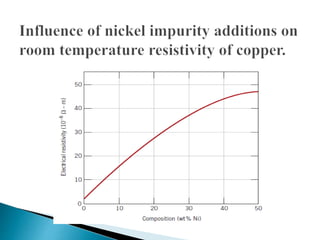
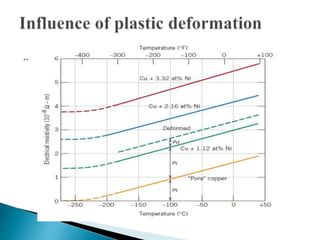
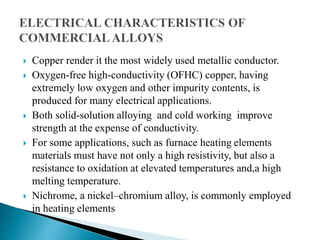
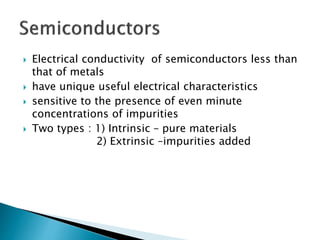
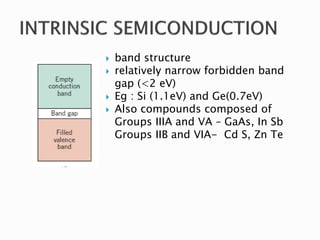
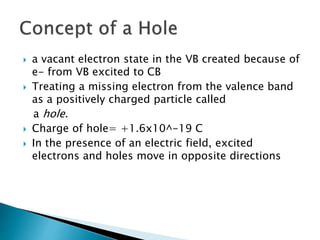
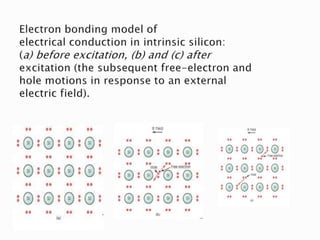
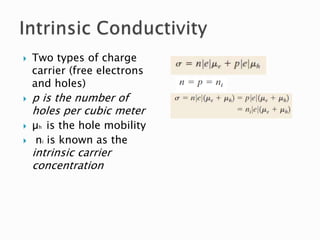
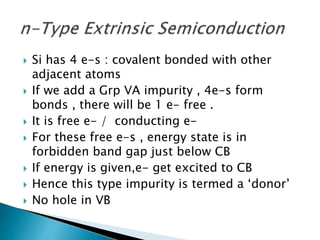
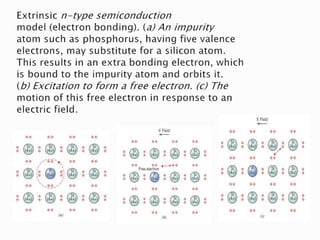
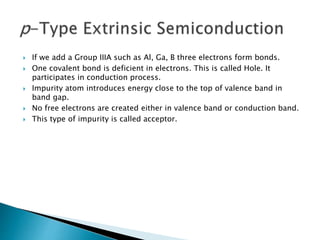
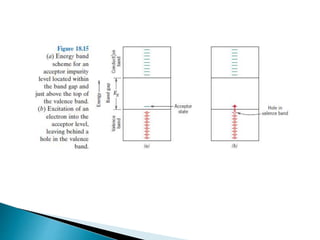
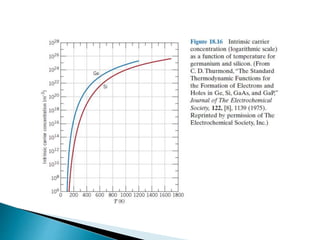
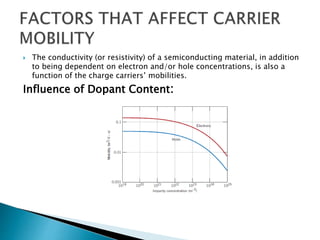
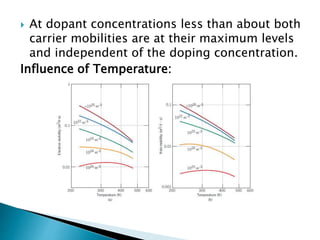
Ohm's law relates current, voltage, and resistance. Resistivity is a material property independent of geometry, while conductivity is the inverse of resistivity and indicates how easily a material conducts electricity. Materials are classified as conductors, insulators, or semiconductors based on conductivity. Semiconductors have applications in electronics due to their sensitivity to impurities and ability to be "doped" to control conductivity. Their band structure results in varying conductivity depending on temperature and doping.


![It is defined as the reciprocal of resistivity
It indicates the case with which a material is capable of conducting
an electric current.
units : σ ( ohm meter (1 ̸Ωm ) )
ohm’s law can be expressed as
where J – current density [I/A]
E – Electric field density ( v/l )
solid materials are classified according to ease with whivh they conduct
electric current . They are classified as
1) conductors : conductivity order
2) insulators
3) semi conductors](https://image.slidesharecdn.com/materialscience-metalsbnadsetc-151105171033-lva1-app6891/85/Material-science-metals-bnads-etc-3-320.jpg)