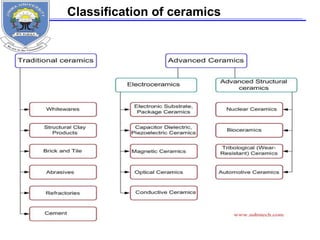
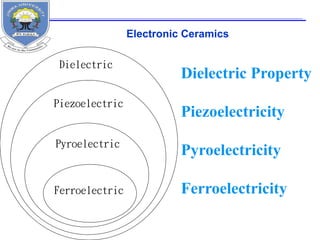
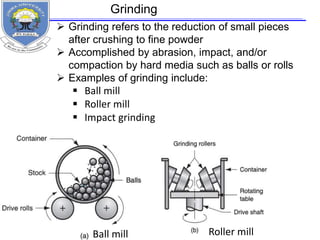
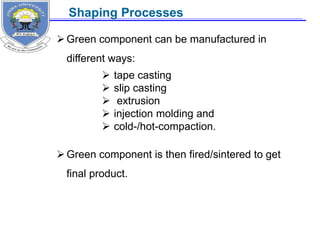
This document provides information on the properties of ceramics. It begins with an introduction to ceramics, including their atomic bonding and crystal structures. It then discusses defects in ceramics and general properties such as brittleness, toughness, and strength at high temperatures. The document classifies ceramics and discusses properties and applications of various types, including electronic ceramics like piezoelectric and dielectric ceramics. Processing methods are also briefly mentioned.














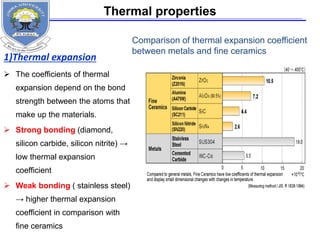



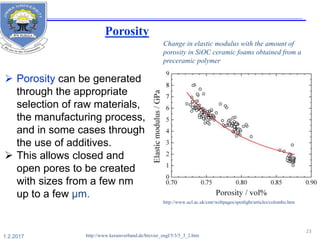
![Elastic modulus
The elastic modulus E [GPa]
of almost all oxide and non-
oxide ceramics is consistently
higher than that of steel.
This results in an elastic
deformation of only about 50
to 70 % of what is found in
steel components.
http://www.keramverband.de/brevier_engl/5/3/4/5_3_4.htm](https://image.slidesharecdn.com/fayzaceramics-170201145338/85/Fayza-ceramics-20-320.jpg)