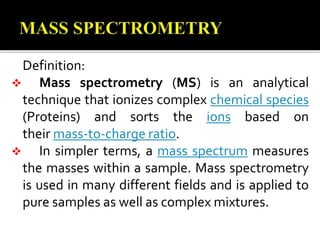
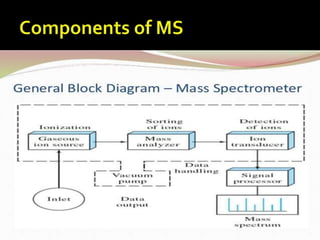
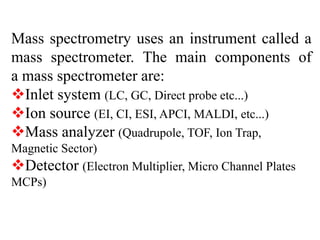
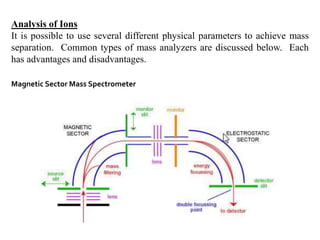
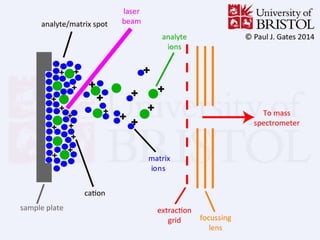
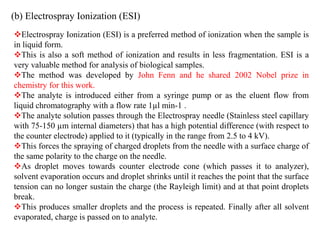
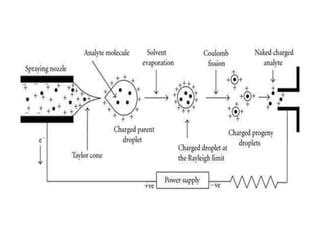
This document provides information about mass spectrometry including definitions, principles, components, and methods of ionization. It defines mass spectrometry as a technique that ionizes chemical species and sorts them by mass-to-charge ratio. The key components of a mass spectrometer are described as the inlet system, ion source, mass analyzer, and detector. Common ionization methods like MALDI and electrospray ionization are explained in terms of how they work to ionize samples for analysis.