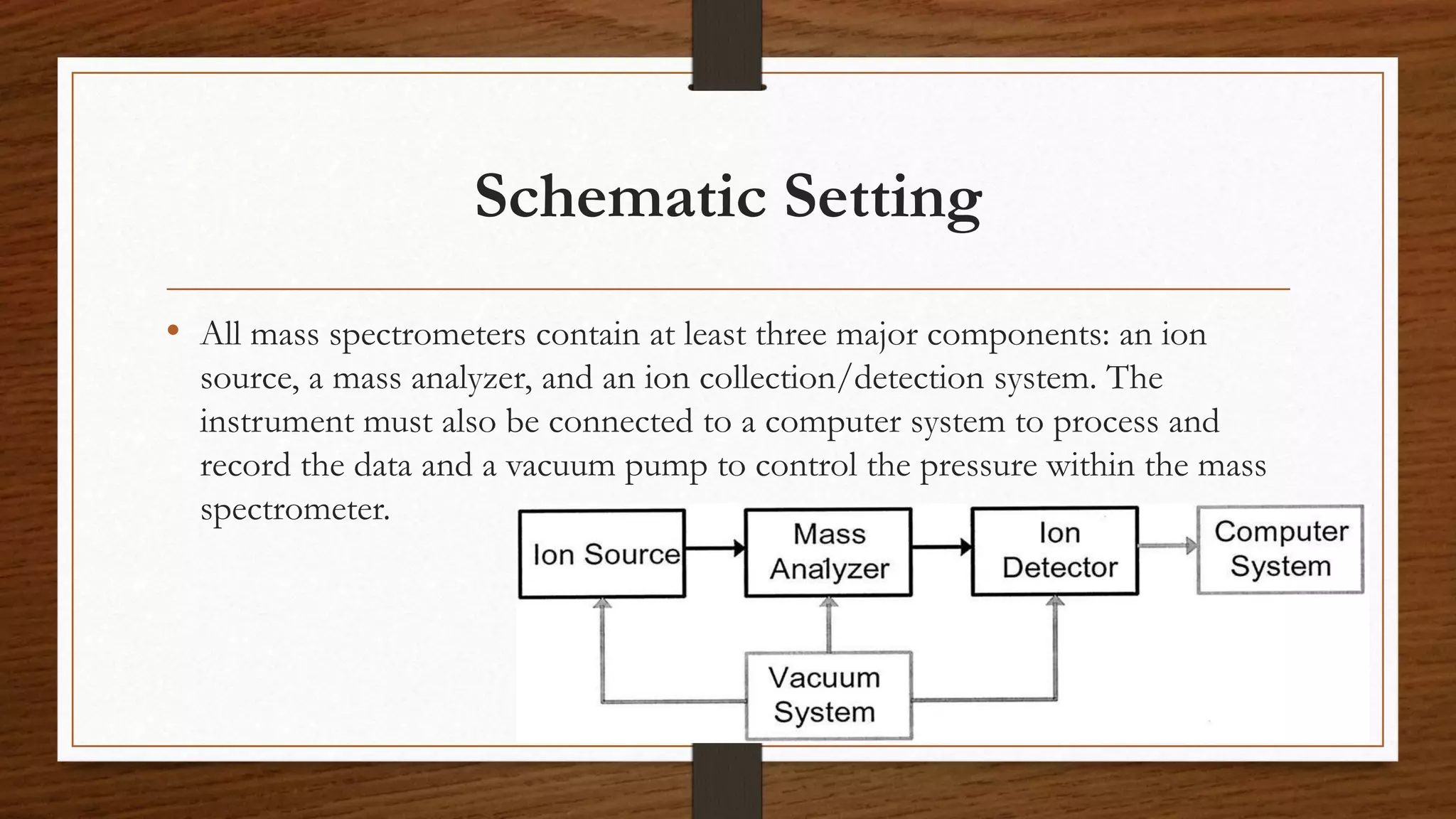
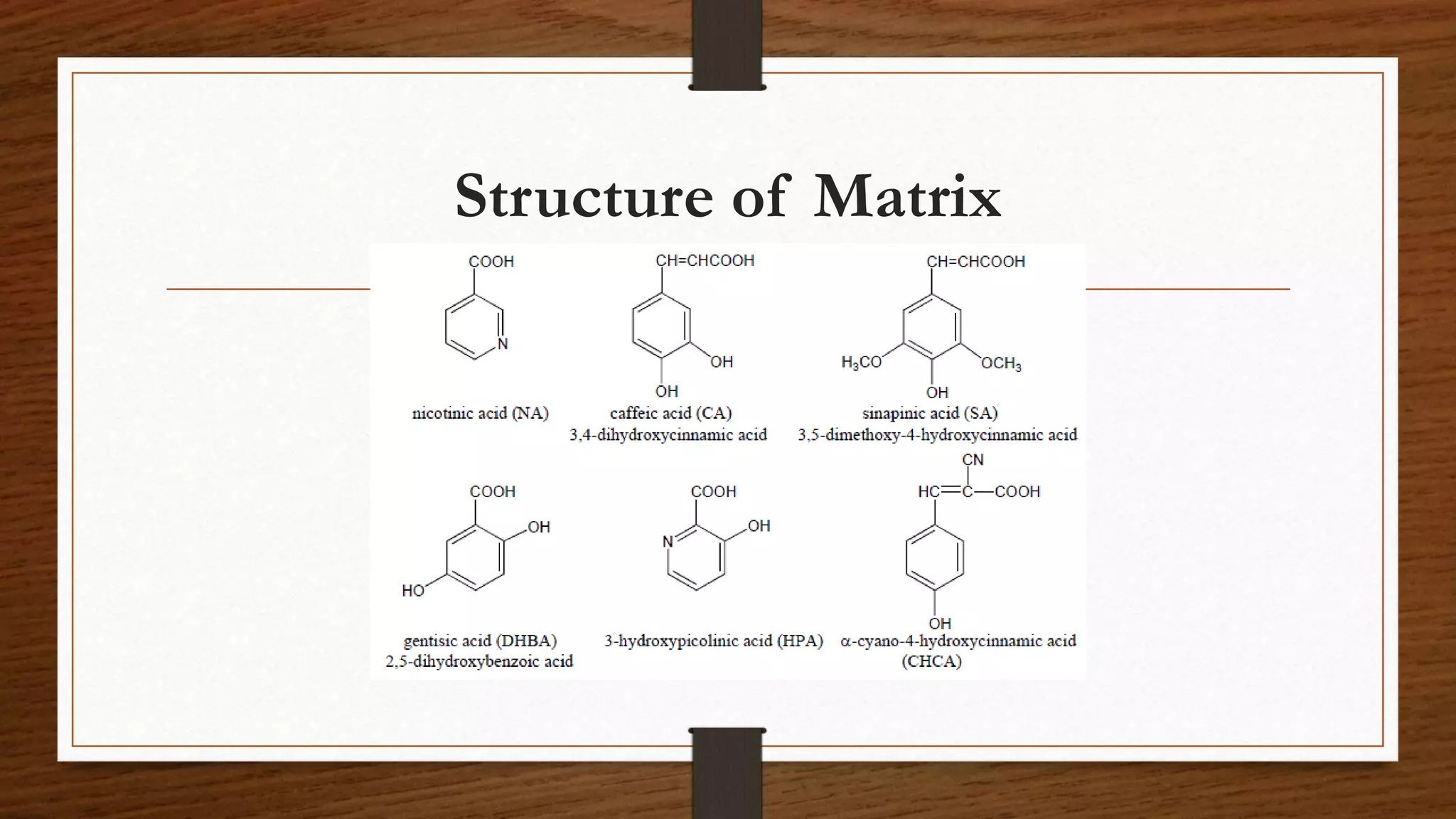
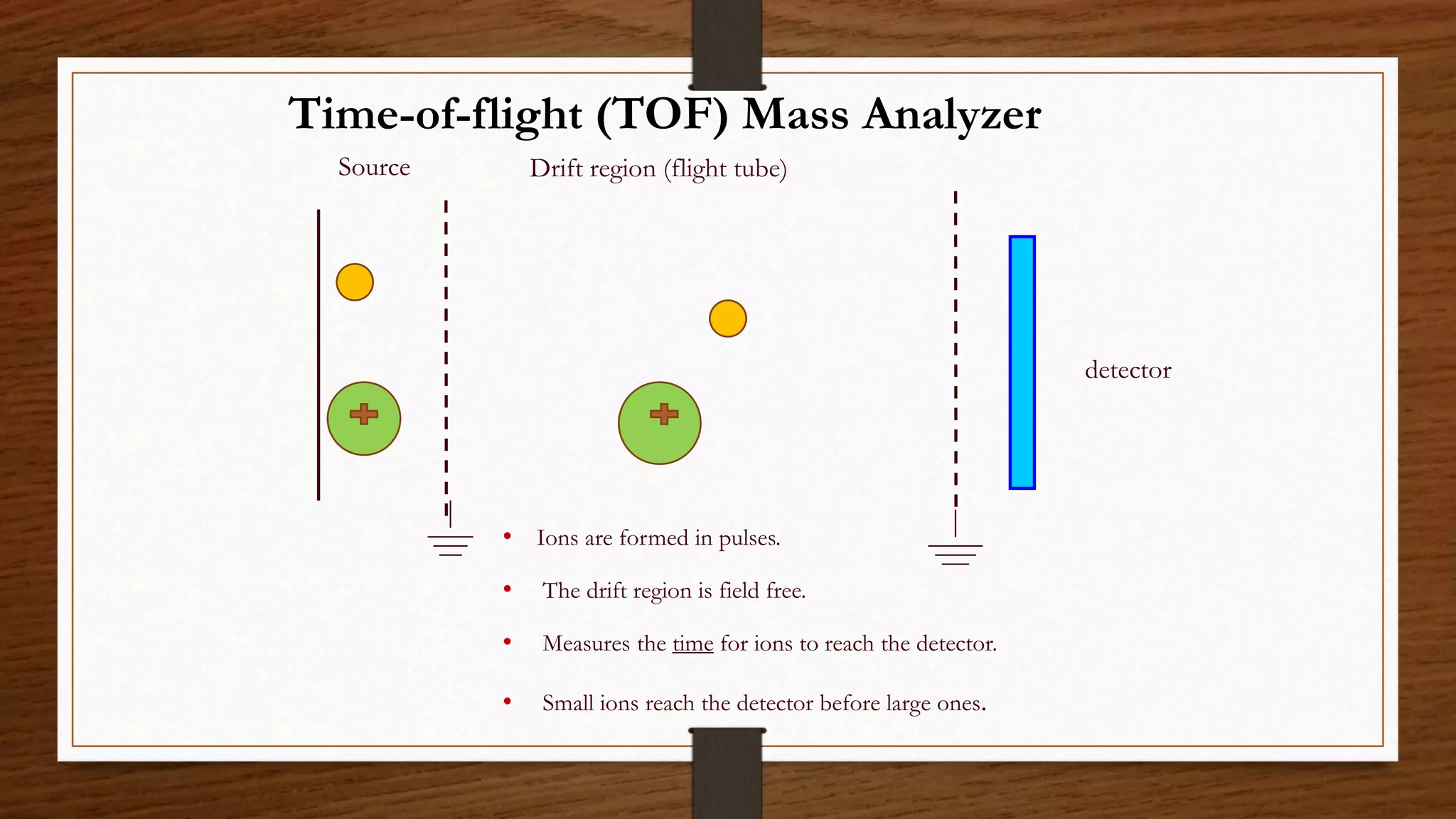
MALDI-TOF mass spectrometry is a soft ionization technique used to analyze biomolecules like proteins, peptides, and polymers. It works by mixing the sample with an organic matrix and applying it to a metal plate. A pulsed laser is used to desorb the sample-matrix mixture, ionizing the analyte via proton transfer. The ions are then analyzed by a time-of-flight mass spectrometer, which measures the time it takes ions to reach the detector based on their mass-to-charge ratio. MALDI-TOF MS has applications in fields like proteomics, microbiology, and pharmaceutical analysis by providing identification and quantification of proteins, metabolites, and microorganisms.