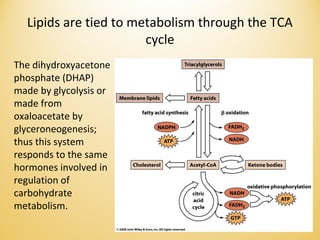
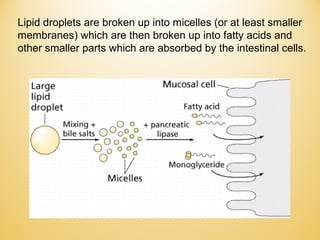
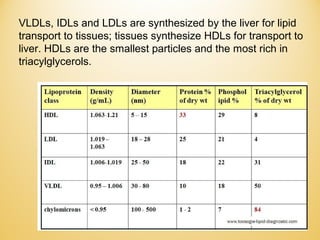
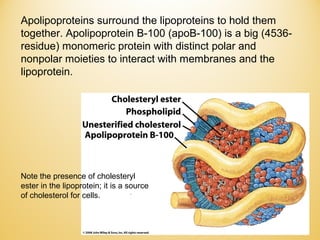
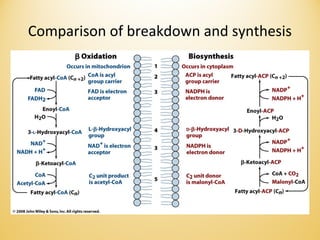
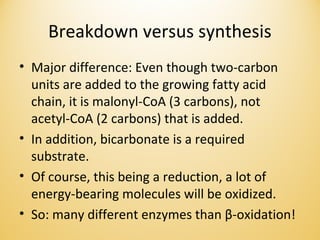
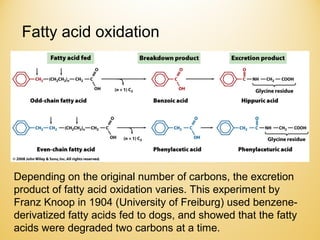
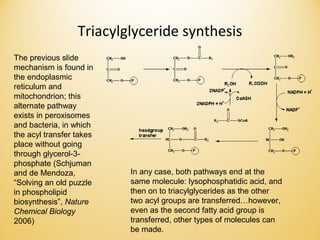
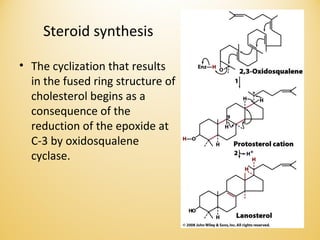
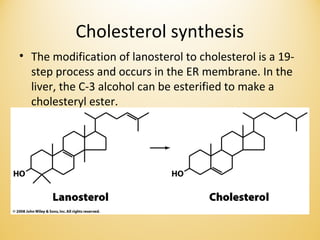
Lipids undergo complex breakdown and biosynthesis pathways. During breakdown, triglycerides are broken down into fatty acids and monoacylglycerols through the action of lipases in the digestive system and transported to tissues. Fatty acids are broken down through beta-oxidation within mitochondria to produce acetyl-CoA. Biosynthesis involves the condensation of malonyl-CoA units to synthesize fatty acids and glycerol-3-phosphate to produce triglycerides, phospholipids, and other lipids. Cholesterol is synthesized from acetyl-CoA through a complex series of reactions involving squalene and lanosterol.