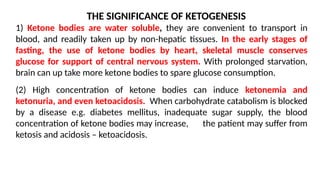
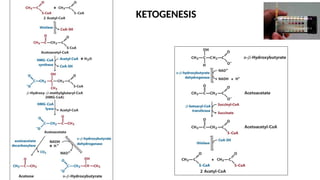
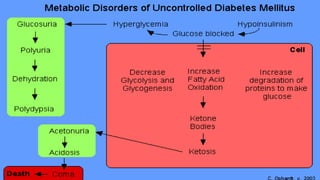
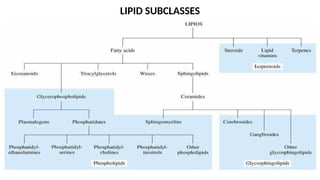
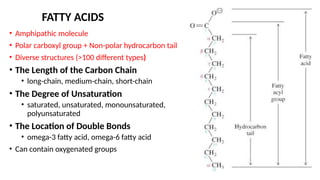
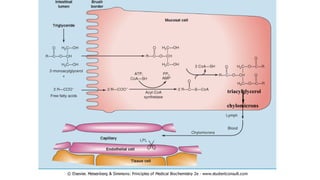
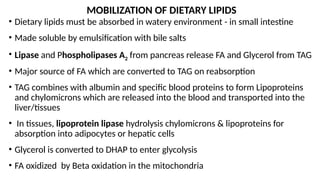
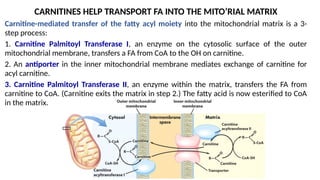
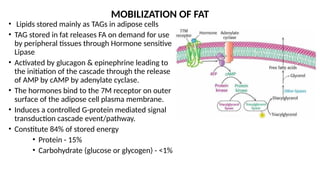
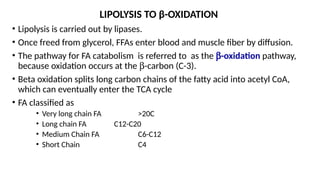
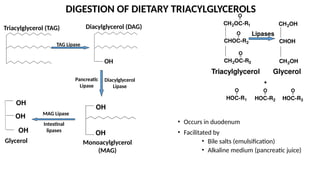
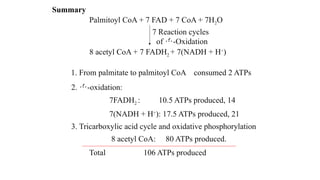
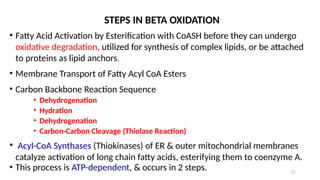
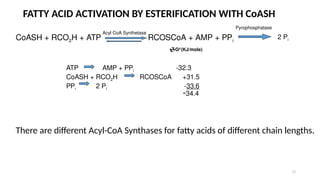
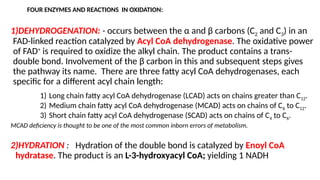
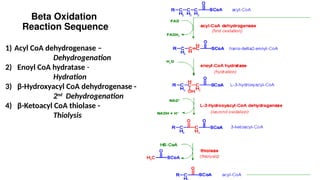
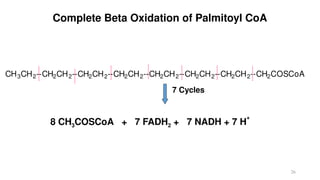
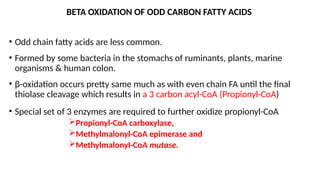
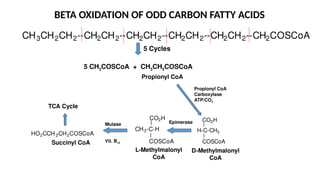
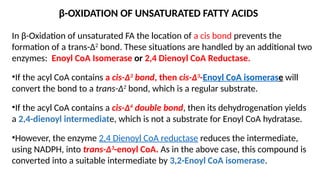
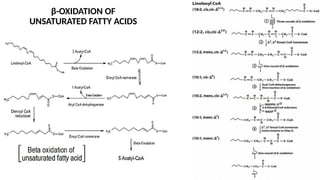
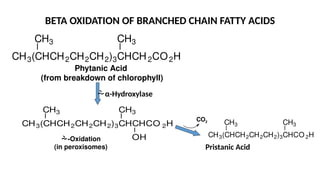
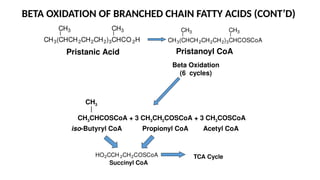
The document outlines the synthesis, oxidation, and classification of lipids, explaining their roles in energy generation and cellular respiration. It details the breakdown of lipids via beta oxidation and the formation of ketone bodies, along with the structure and function of various lipid subclasses. The text also discusses fatty acid metabolism, including activation, transport, and the enzymatic processes involved in their catabolism.








![CONTROL OF FATTY ACID OXIDATION
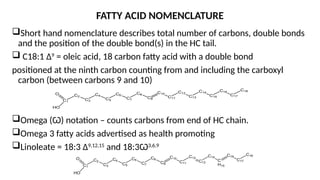
Control of fatty acid oxidation is exerted mainly at the step of FA entry into mitochondria.
Malonyl CoA (which is also a precursor for fatty acid synthesis) inhibits Carnitine Palmitoyl
Transferase I. Malonyl-CoA is produced from acetyl-CoA by the enzyme Acetyl-CoA
Carboxylase.
AMP-Activated Kinase, a sensor of cellular energy levels, is allosterically activated by AMP,
which is high in concentration when [ATP] is low.
Acetyl-CoA Carboxylase is inhibited when phosphorylated by AMP-Activated Kinase, leading
to decreased Malonyl-CoA.
The decrease in Malonyl-CoA concentration leads to increased activity of Carnitine Palmitoyl
Transferase I.
Increased fatty acid oxidation then generates acetyl-CoA, for entry into Krebs cycle with
associated ATP production](https://image.slidesharecdn.com/lecture9betaoxidation-241116112006-64c19dac/85/LECTURE-Note-on-Lipid-Metabolism-Oxidation-ppt-34-320.jpg)