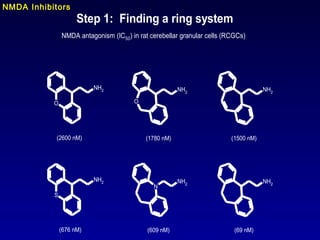
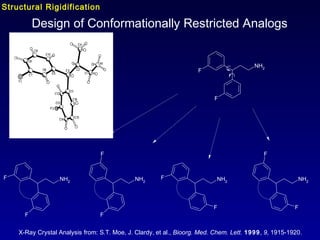
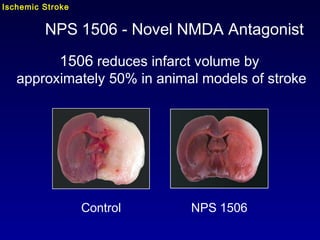
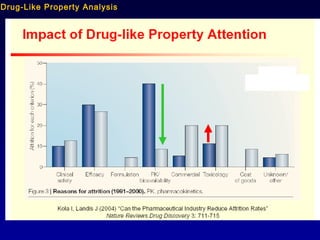
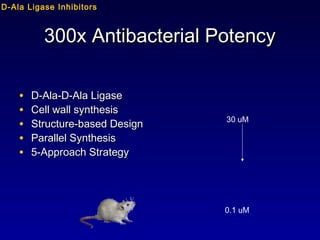
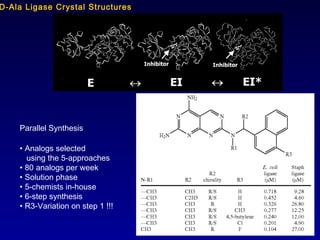
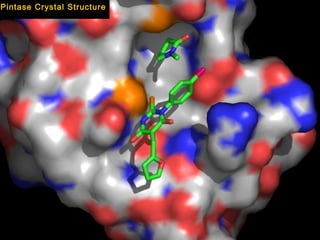
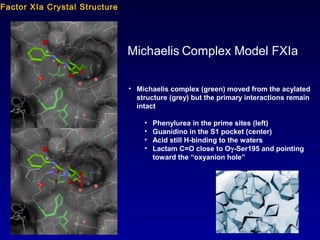
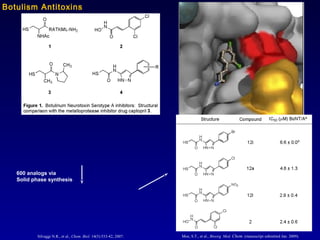
This document summarizes research from several publications investigating compounds that act on serotonin, dopamine, opioid, and calcium receptor systems. It describes the synthesis and evaluation of analogs of aporphine, an alkaloid, examining their effects on serotonin and dopamine receptors. It also discusses the development of opioid receptor agonists and antagonists through stereospecific synthesis and evaluation of derivative compounds. Finally, it mentions the development of cinacalcet and other calcium sensing receptor modulators for the treatment of hyperparathyroidism.
![Portoghese, P.S., S. Ohkawa, and S.T. Moe. Preparation of spiroindane opiate analogs; 1994. U. S. Pat. 5,298,622
Kshirsagar, T.A., S.T. Moe, and P.S. Portoghese. Stereospecific synthesis of pseudocodeine: [2,3]-Sigmatropic rearrangement using selenium intermediates. J. Org. Chem. 63(5), 1704-1705, 1998.
Portoghese, P.S., S. Ohkawa, S.T. Moe, and A.E. Takemori. Synthesis and delta-opioid receptor antagonist activity of naltrindole analogues with a regioisomeric indole moiety. J. Med. Chem. 37(12), 1886-1888, 1994.
Portoghese, P.S., M. Sultana, S.T. Moe, and A.E. Takemori. Synthesis of naltrexone-derived δ-opioid antagonists. Role of conformation of the δ address moiety. J. Med. Chem. 37(5), 579-585, 1994.
Portoghese, P.S., S.T. Moe, and A.E. Takemori. A selective delta-1 opioid receptor agonist derived from oxymorphone. Evidence for separate recognition sites for delta-1 opioid receptor agonists and antagonists. J. Med. Chem. 36(17), 2572-2574, 1993.
Kong, H., K. Raynor, K. Yasuda, S.T. Moe, P.S. Portoghese, G.I. Bell, and T. Reisine. A single residue, aspartic acid 95, in the δ-opioid receptor specifies selective high-affinity agonist binding. J. Biol. Chem. 268(31), 23055-23058, 1993.
morphine
enkephalin
Opioid Antagonists](https://image.slidesharecdn.com/moe-websiteslides-101122005532-phpapp02/85/Leadops-History-2-320.jpg)