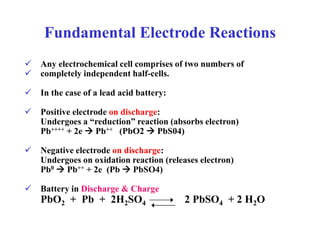
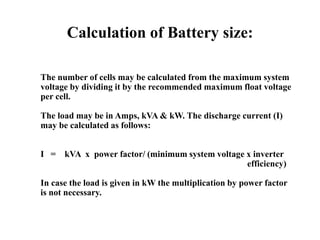
The document provides an overview of lead acid batteries, including their components, applications, types, selection criteria, sizing calculations, maintenance practices, troubleshooting instructions, and safety precautions. Key information includes how to choose the right battery based on application requirements, as well as detailed maintenance procedures to ensure longevity and efficiency. It emphasizes the importance of safety when handling batteries and disposing of hazardous materials.