Le Chatelier's Principle states that if a system at equilibrium is stressed, it will shift to relieve the stress and reestablish equilibrium. The document discusses four types of stresses - concentration changes, temperature changes, pressure/volume changes, and the presence of a catalyst - and how systems respond to each to reestablish equilibrium. It also provides examples of how Le Chatelier's Principle applies to batteries, oxygen transport in hemoglobin, and industrial processes.



![1. Concentration Stress
Stress: a change in concentration
of products or reactants by adding or
removing.
Adjustment: change in collision rate and
redistribution of particles.
• [Add] – system shifts to use it up.
• [Remove] – system shifts to make more.](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-5-320.jpg)
![• More C means increased rate of reverse reaction.
Kc = [C]
[A][B]
C
B
A +
Kc = 1.35
We say “shifts left”
We mean:
• Excess C used up until ratio of product to reactant
concentrations is equal to Kc once again.
Increase [C]:](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-6-320.jpg)
![Kc = [C]
[A][B]
B C
A +
Kc = 1.35
• Forward reaction is favoured
We say “shifts right”
We mean:
• New concentrations re-establish Kc.
Increase [B]:](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-7-320.jpg)
![Kc = [C]
[A][B]
B C
A +
Kc = 1.35
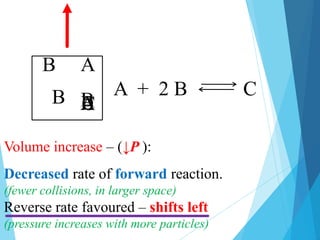
Removing a particle is like decreasing [ ].
• Decreased rate of forward reaction collisions.
We say “shifts left”
We mean:
• Reverse is favoured, ↑ reactants, Kc the same.
Decrease [A]:](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-8-320.jpg)
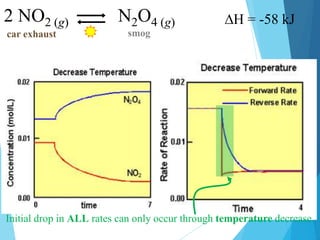
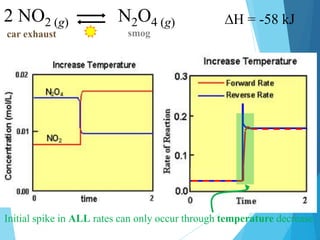
![2 NO2 (g) N2O4 (g)
car exhaust smog
Huge spike indicates that [ ] was changed by adding more particles.](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-10-320.jpg)
![2 NO2 (g) N2O4 (g)
car exhaust smog
A huge spike indicates that [ ] was changed by removing particles.](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-11-320.jpg)
![Temperature stress addressed the SAME way as
concentration by changing collision rates.
**Re-establishes new eqlbm (with new [ ]s)
at new temperature – SO…changes the Kc.
Exothermic: A B (- ∆H )
Endothermic: A B (+ ∆H)
HEAT +
+ HEAT
2. Temperature stress](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-13-320.jpg)
![Temperature increase / add heat
• Reaction shifts left.
• Endothermic collisions (reverse) favored.
Temperature decrease / removing heat
• Reaction shifts right.
• Exothermic collisions (forward) favored.
+ heat
heat
A B
+
A B
= [B]
[A]
= [B]
[A]
Kc
Kc](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-14-320.jpg)








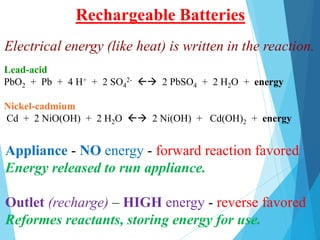
![Haemoglobin protein used to transport O2 from lungs to
body tissue.
Lungs - [O2] is high - forward reaction favored
Haemoglobin binds O2
Tissue - [CO2] is high and [O2] is low - reverse
reaction favored. Hb releases O2
Hb (aq) + O2 (g) HbO2 (aq)
Haemoglobin AND Oxygen](https://image.slidesharecdn.com/lechateliersprinciple2-231226173408-42d3792f/85/Le-Chateliers-Principle-2-chemistry-grade-12-29-320.jpg)
