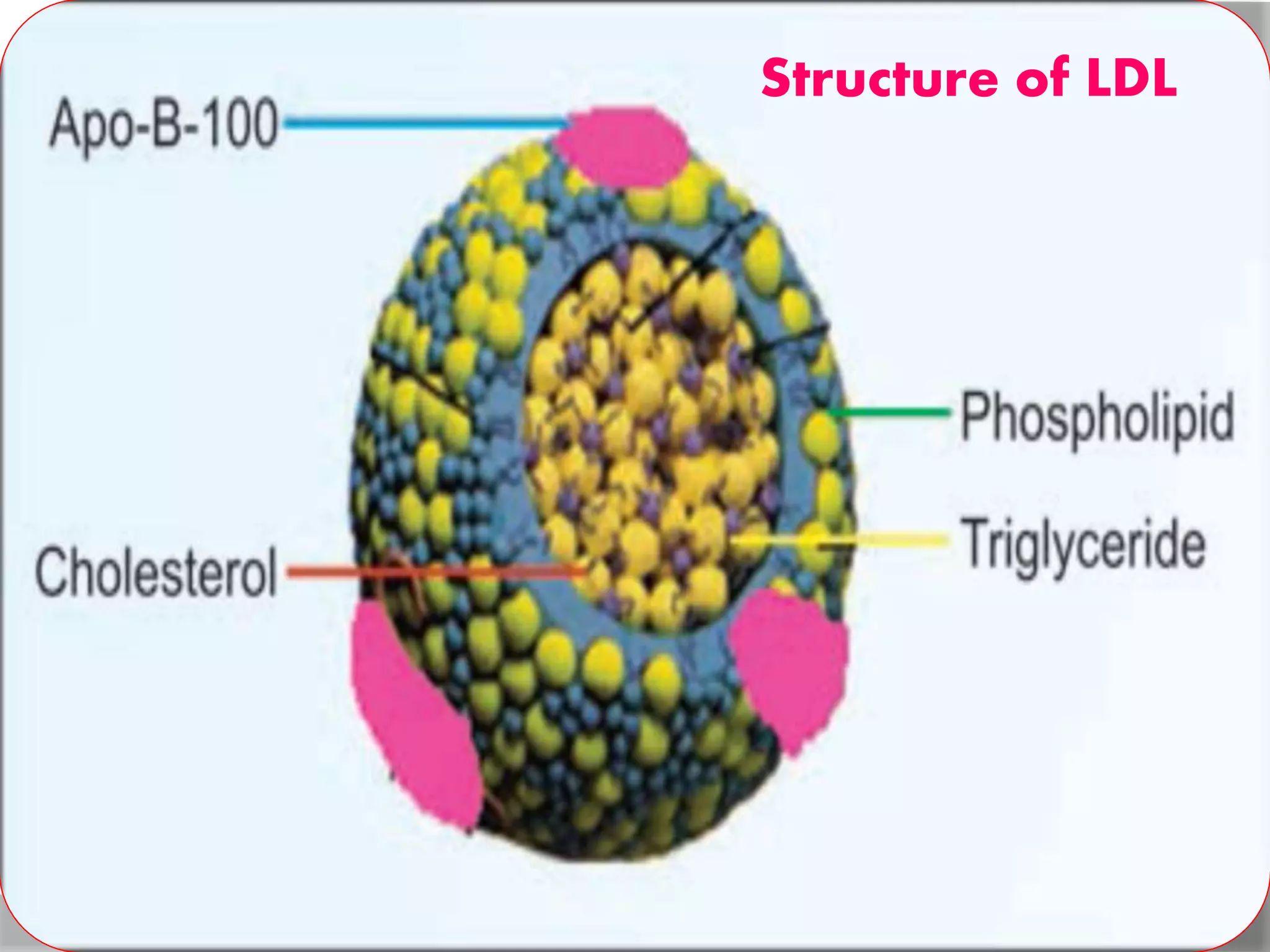
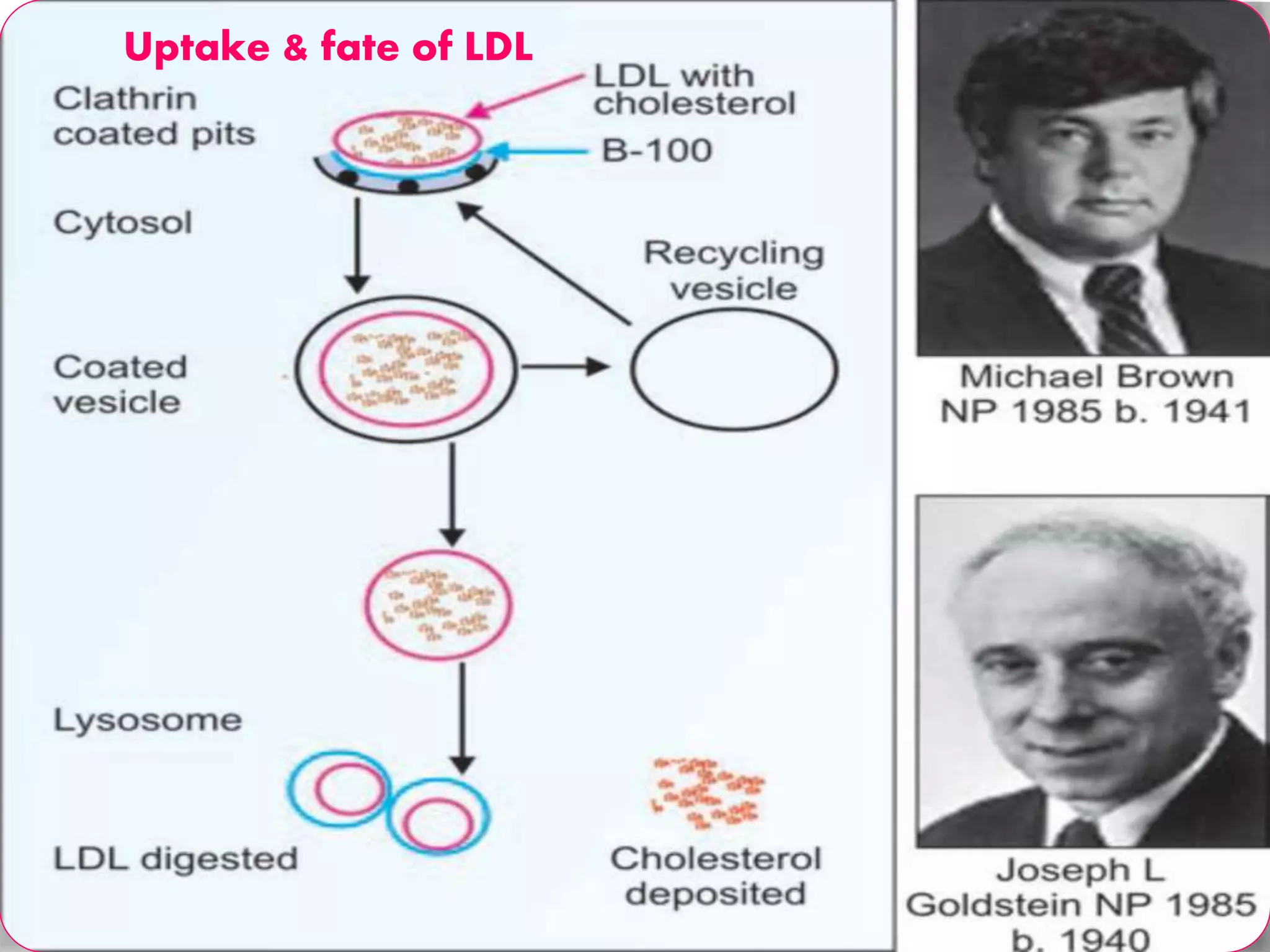
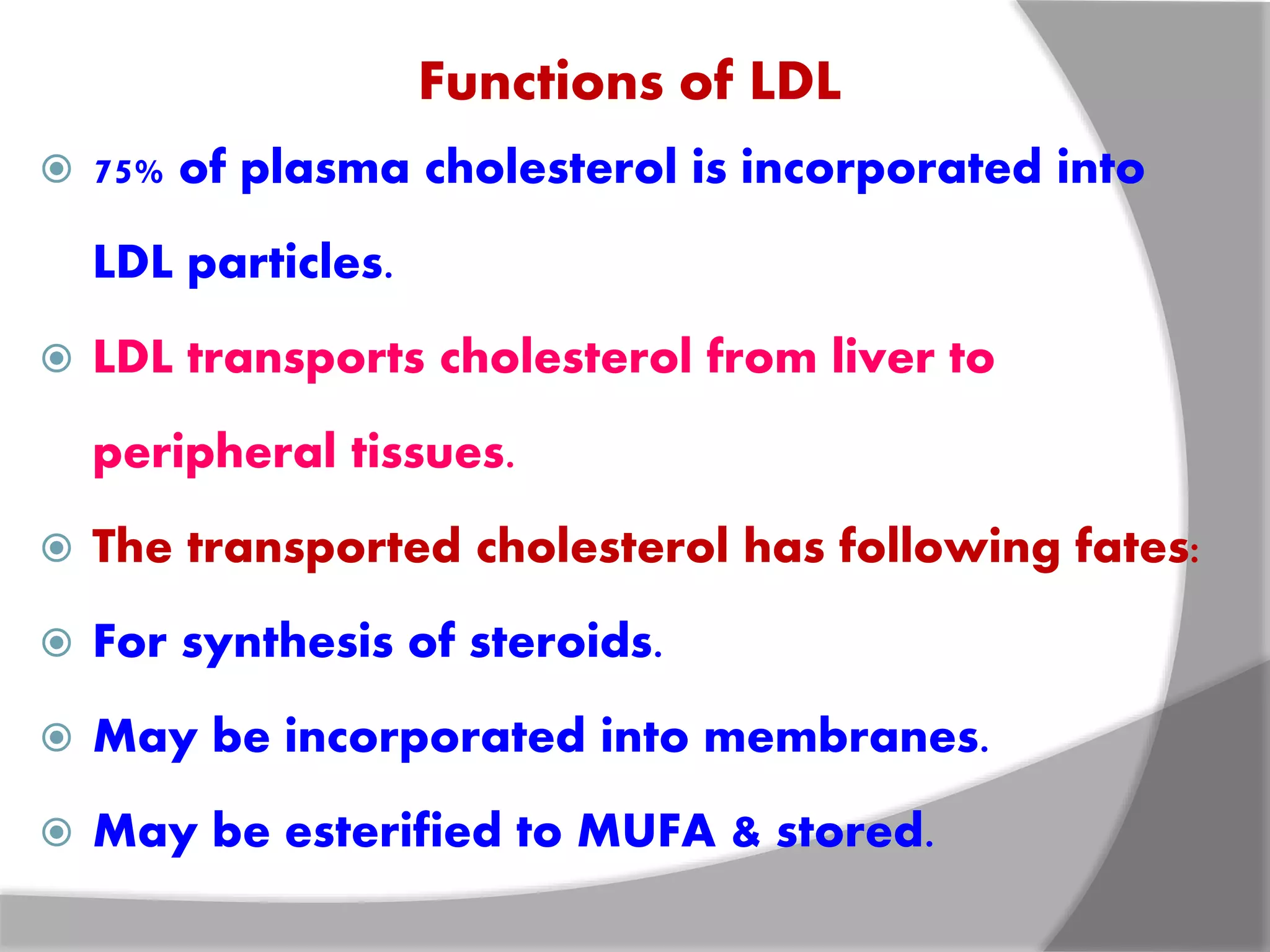
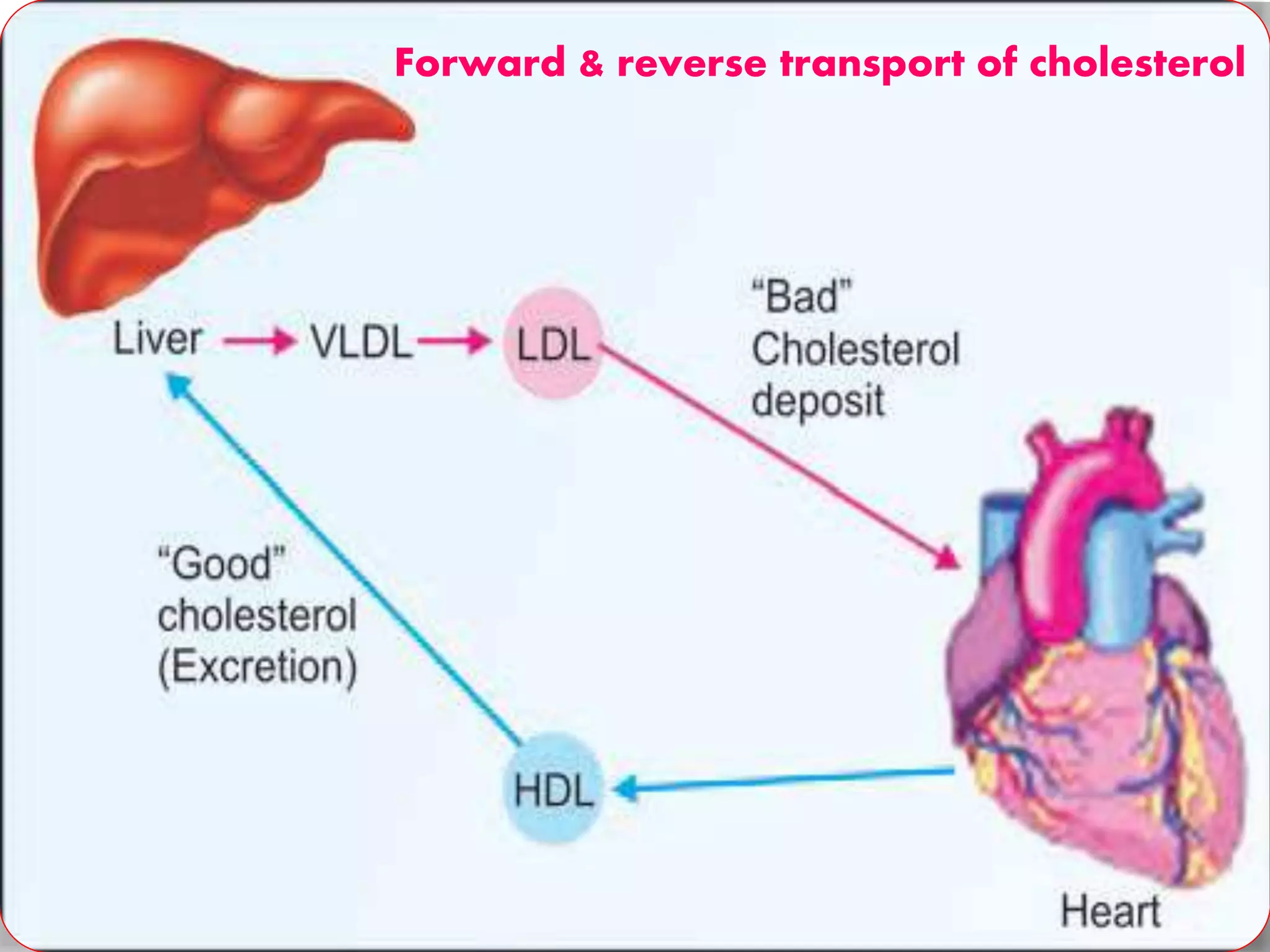
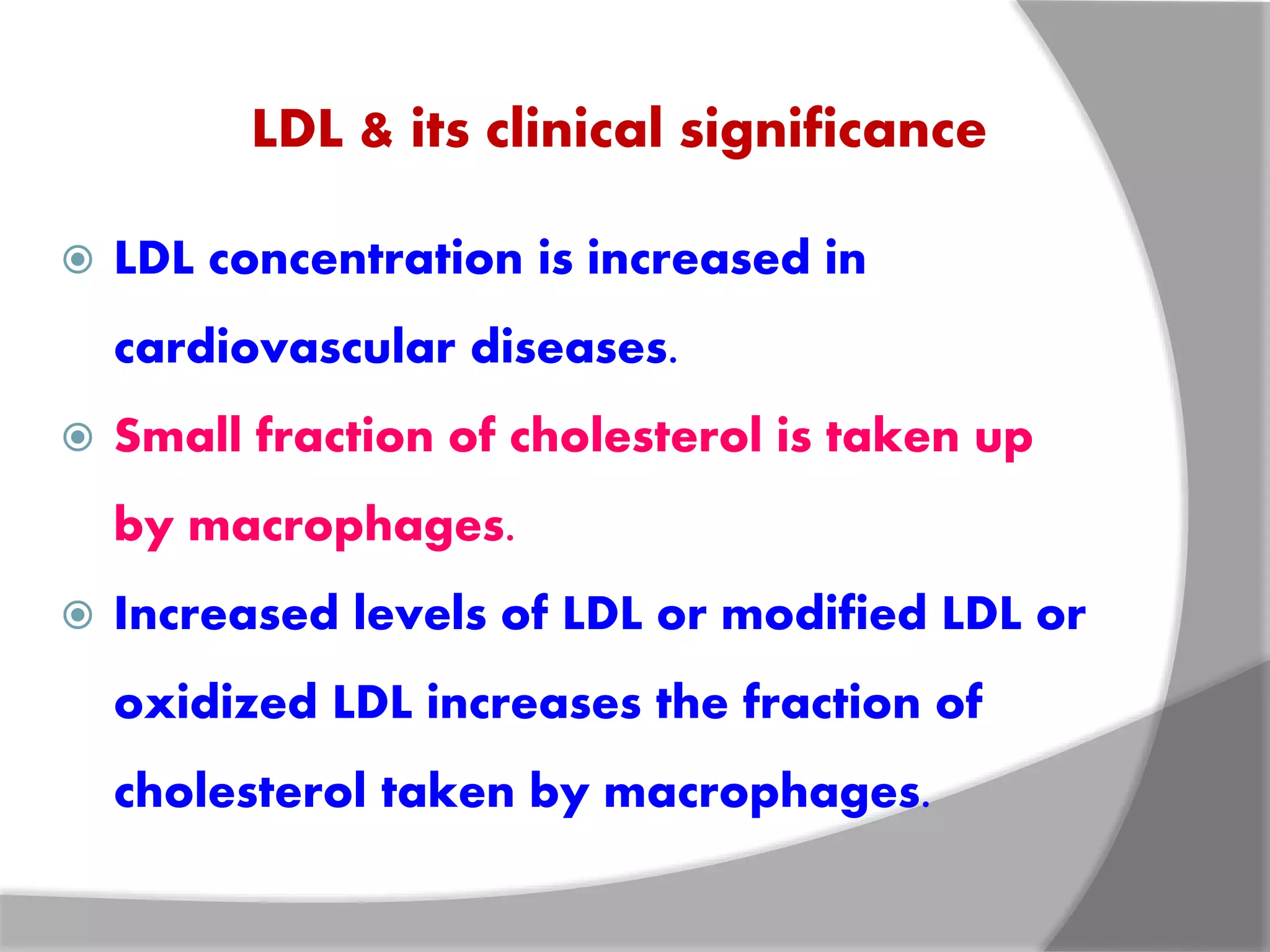
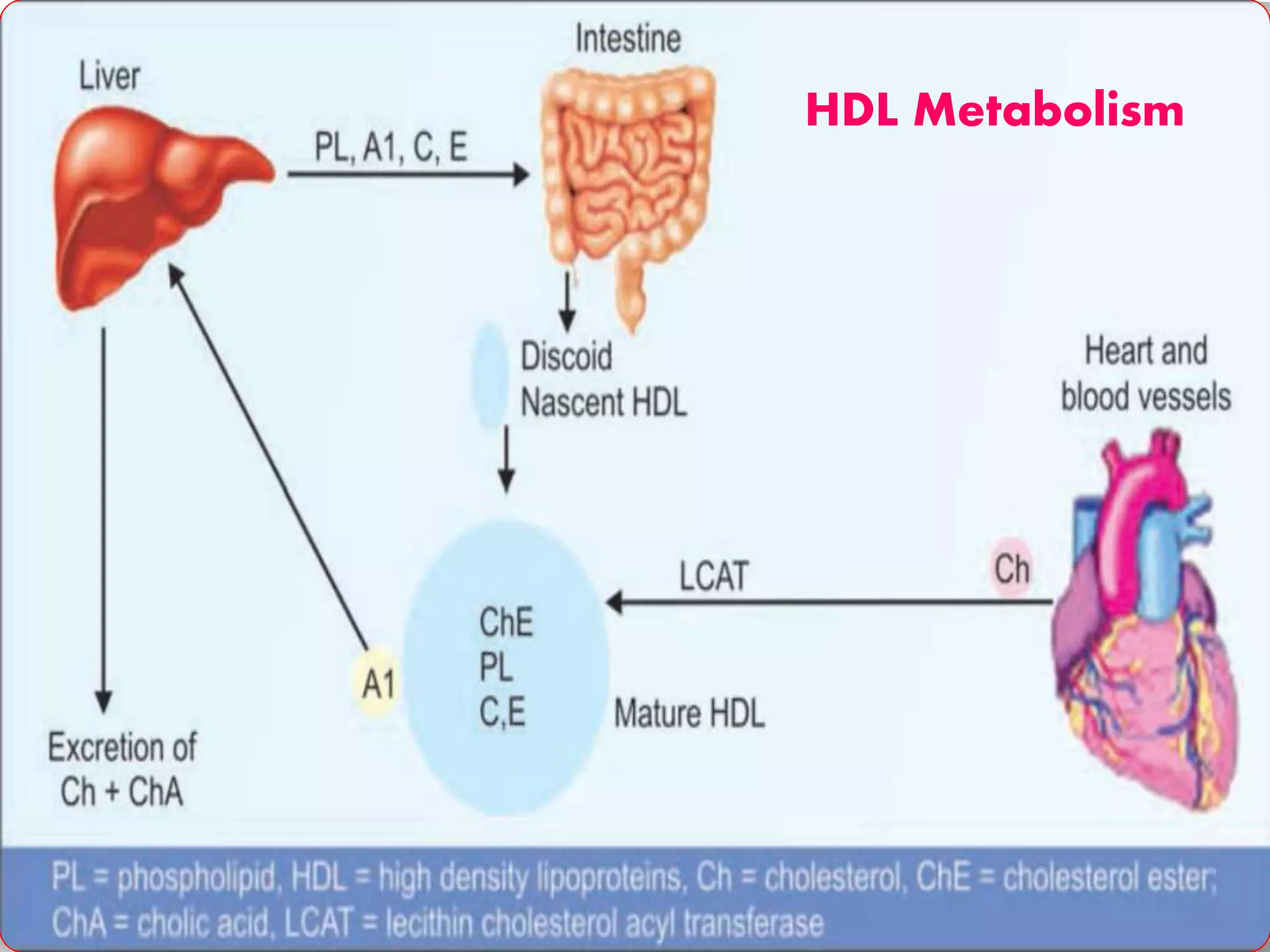
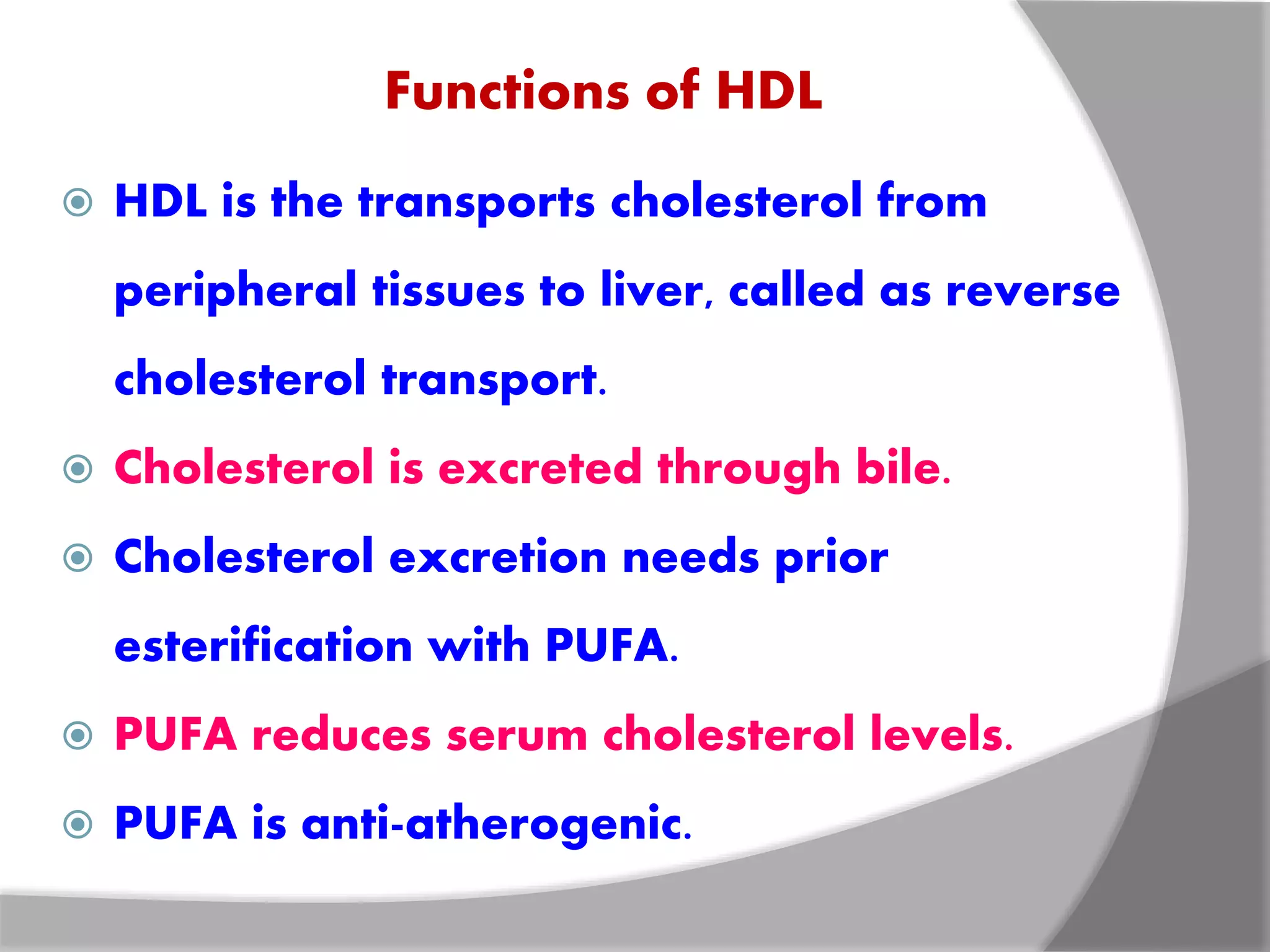
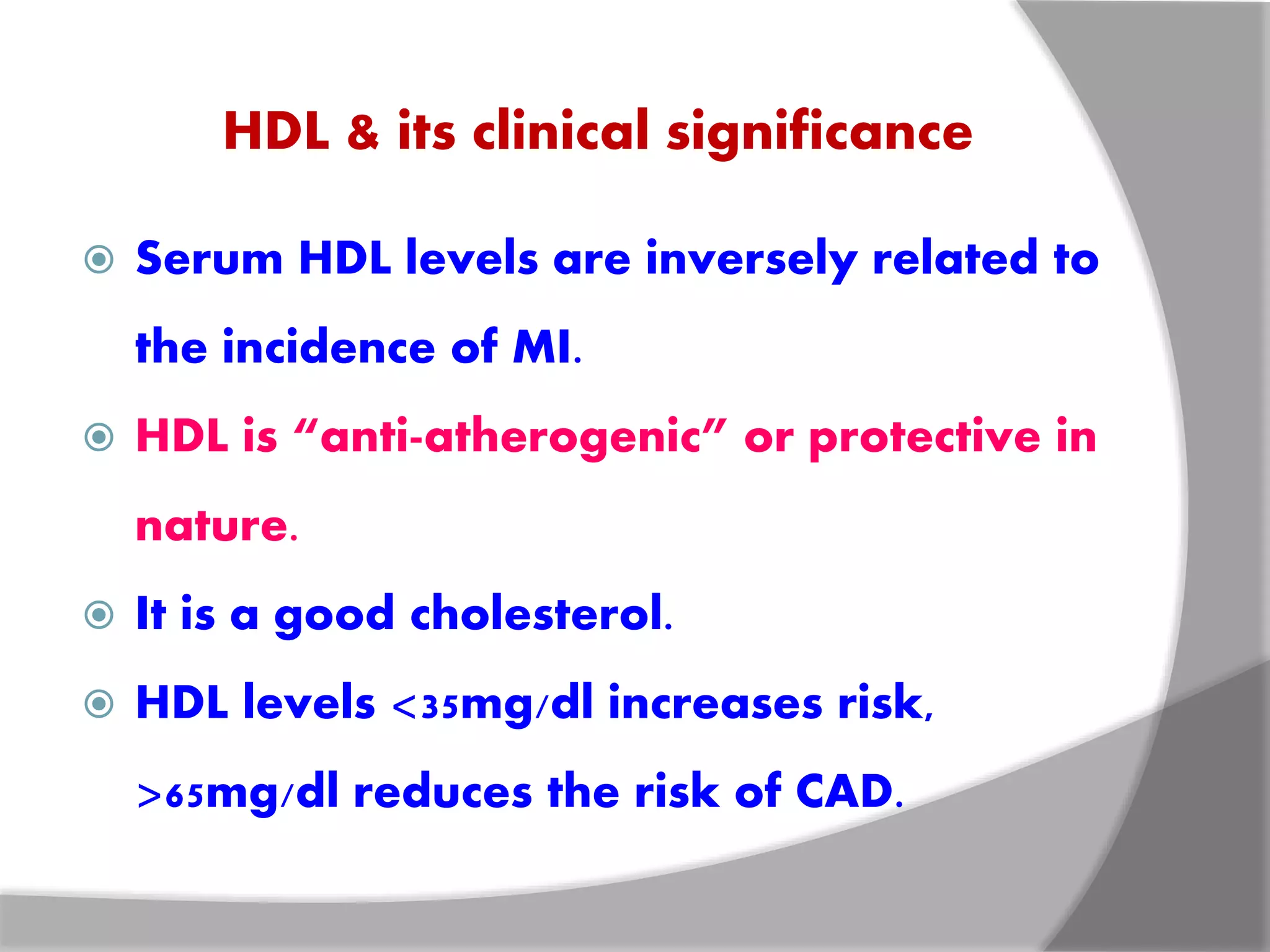
This document discusses LDL metabolism and structure. It notes that LDL transports cholesterol from the liver to tissues, contains one apolipoprotein, and has a half-life of 2 days. LDL is taken up by tissues through receptor-mediated endocytosis, and defects in LDL receptors can lead to familial hypercholesterolemia. High LDL levels are a risk factor for cardiovascular disease by contributing to atherosclerotic plaque formation. The document also summarizes HDL metabolism and functions, noting it transports cholesterol from tissues to the liver in a reverse transport process.