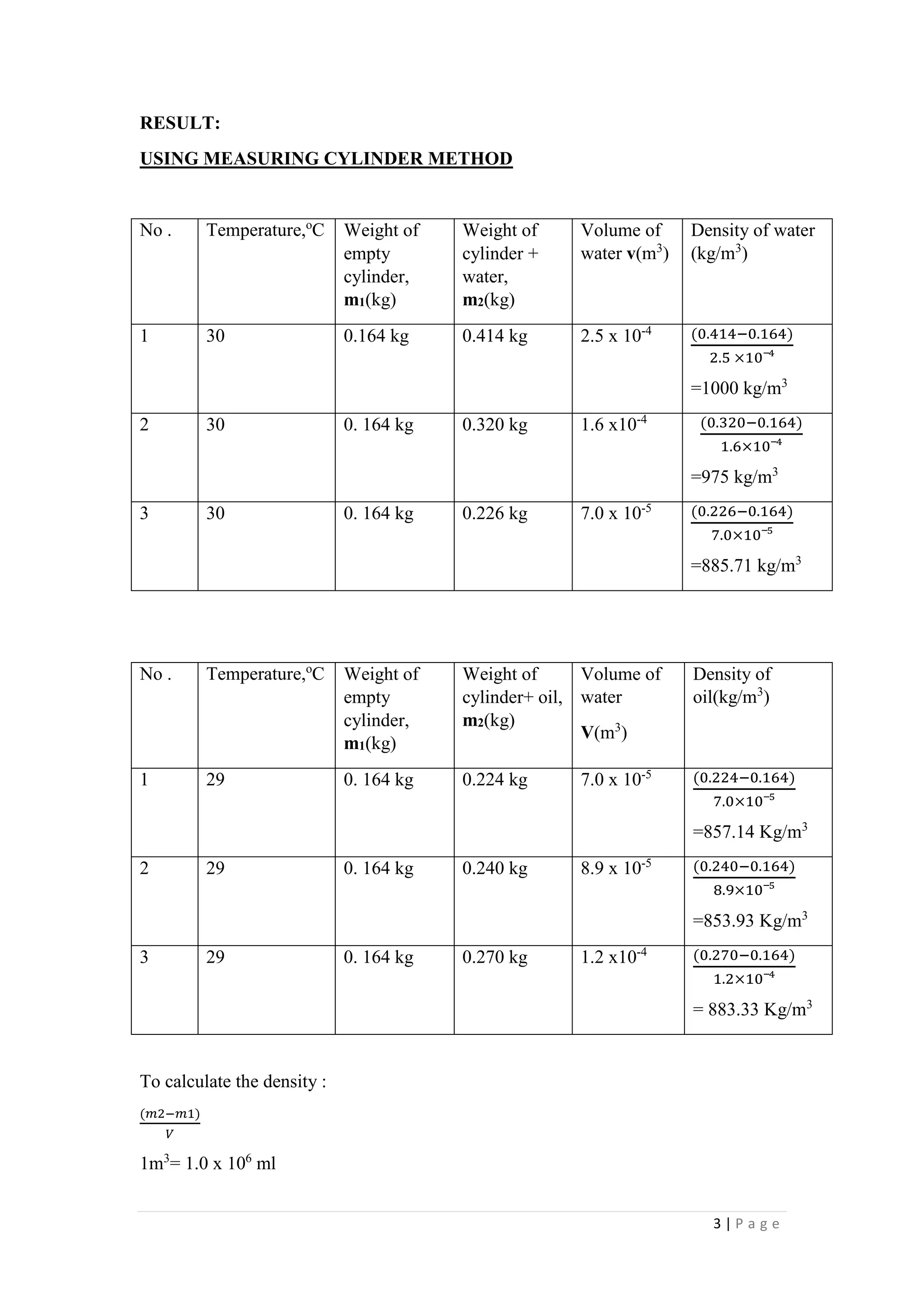
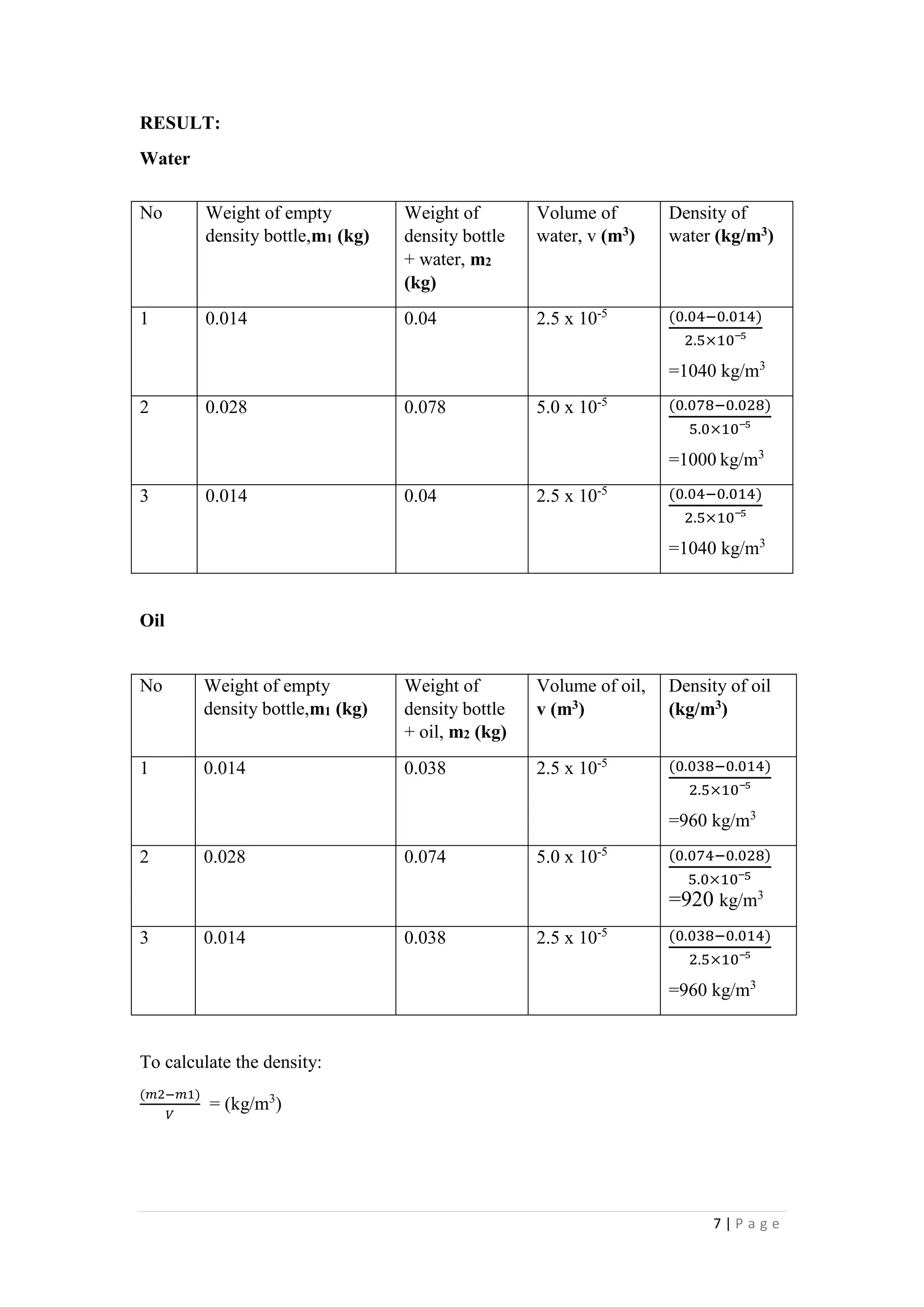
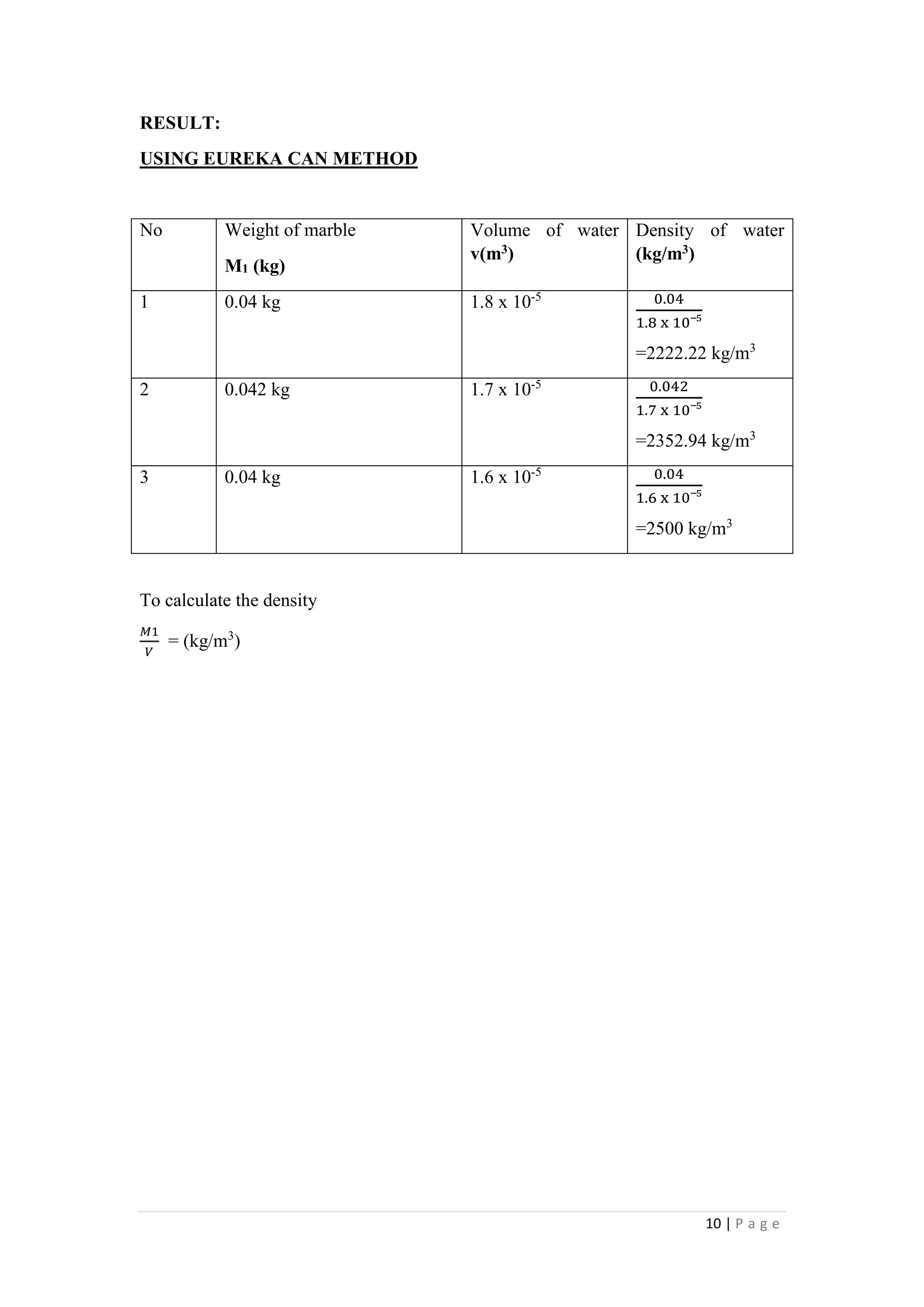
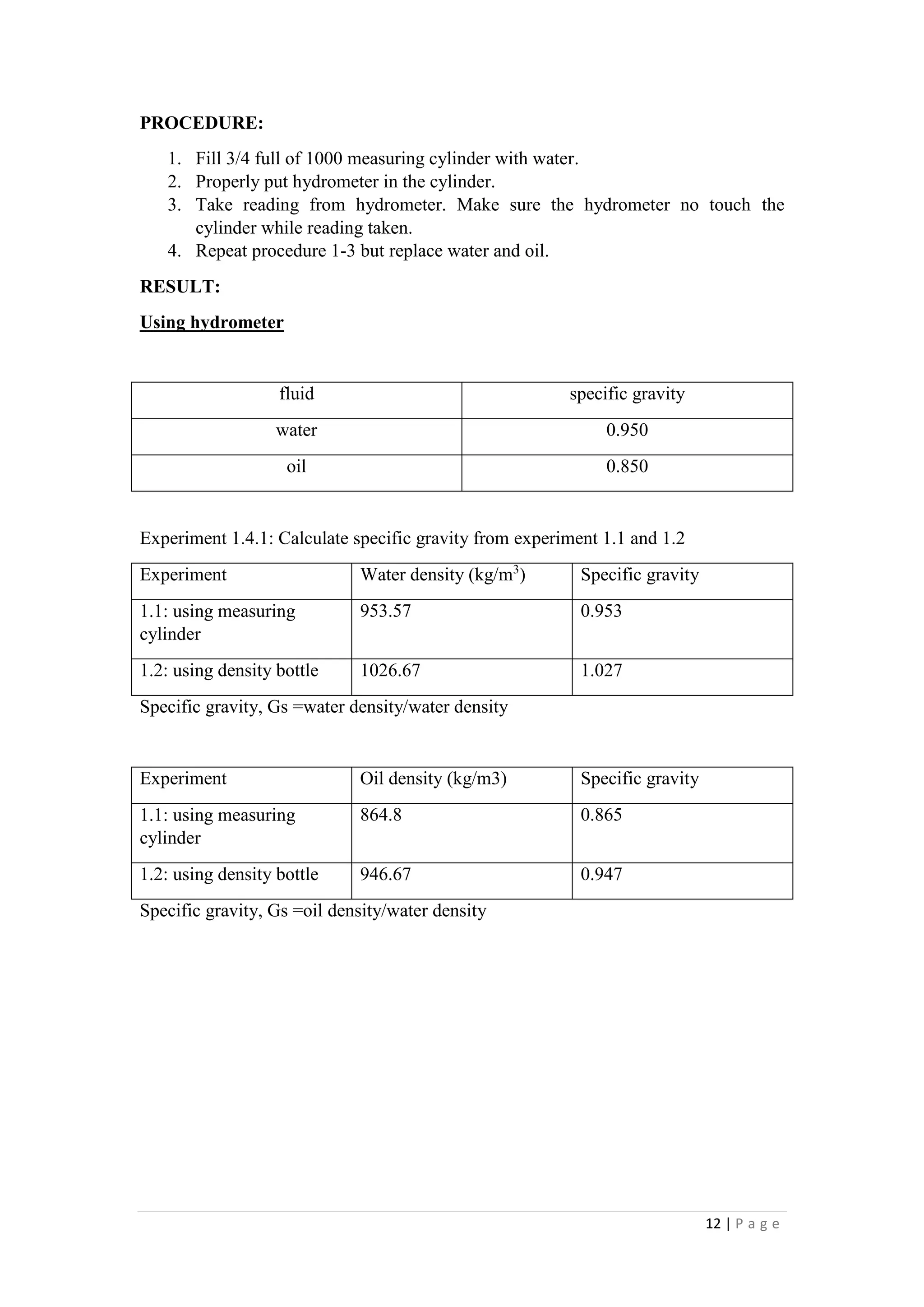
1. The document describes four experiments to determine the density of water and oil using different methods: a measuring cylinder, density bottle, Eureka can, and hydrometer.
2. The densities measured ranged from 885-1000 kg/m3 for water and 857-883 kg/m3 for oil depending on the method. The density bottle was deemed the most accurate method.
3. Specific gravities were also calculated from the density measurements, with water having a specific gravity of 0.953-1.027 and oil 0.865-0.947.