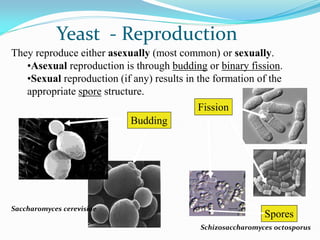
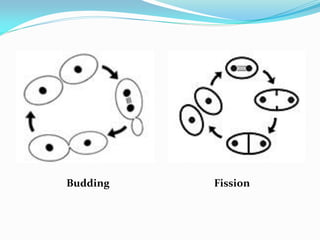
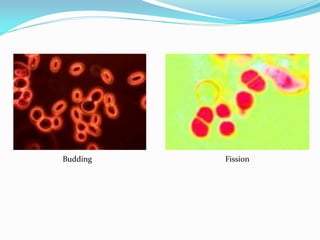
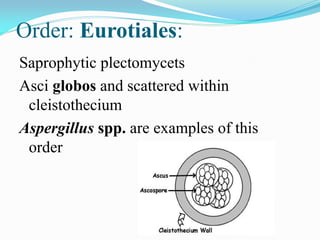
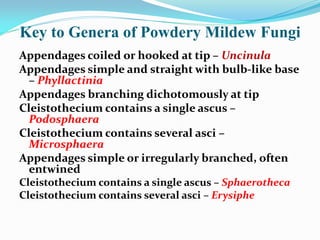
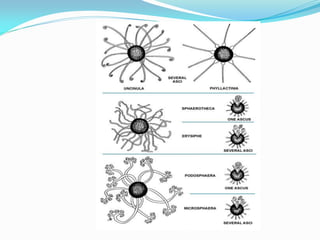
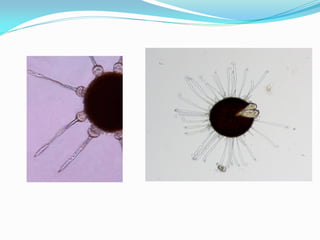
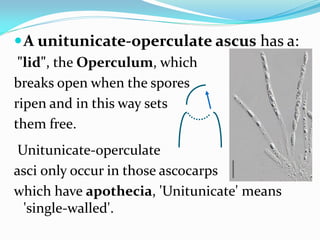
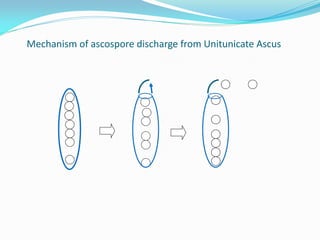
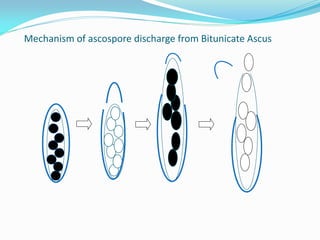
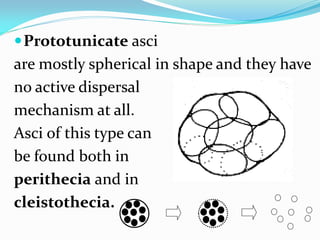
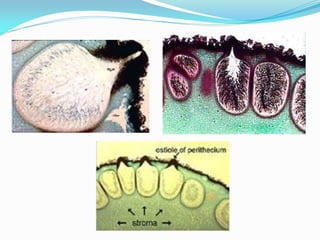
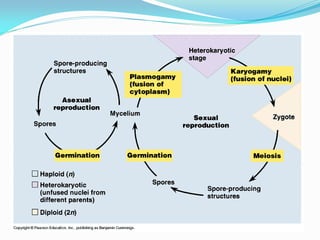
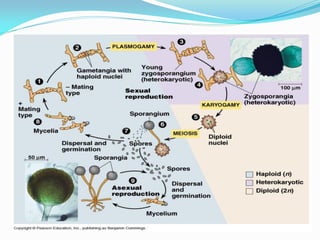
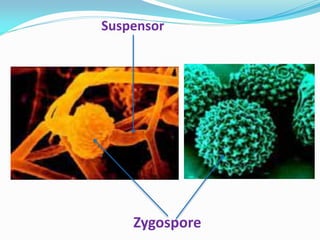
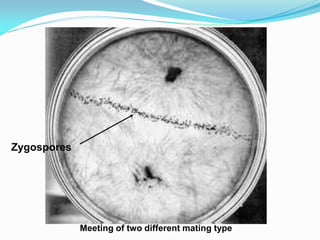
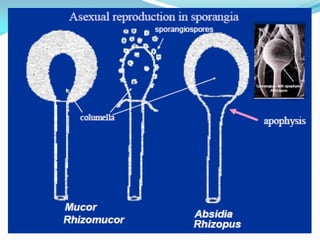
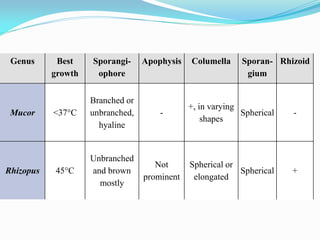
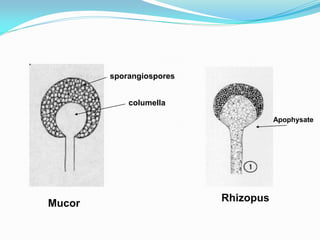
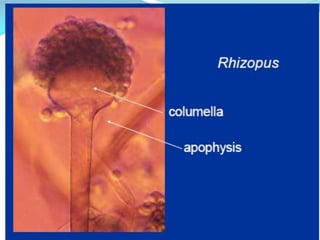
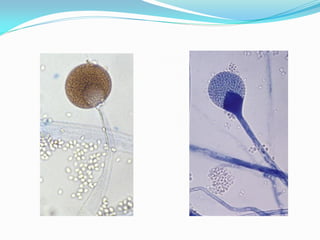
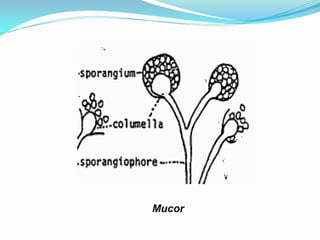
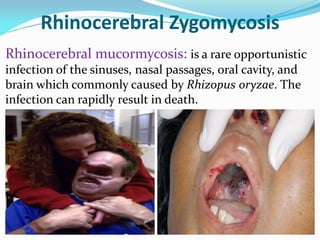
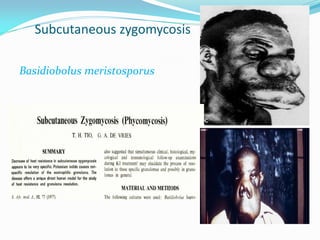
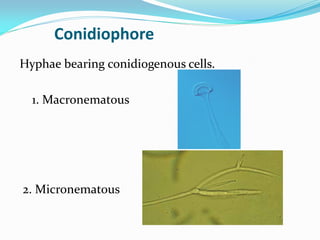
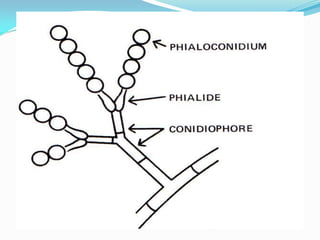
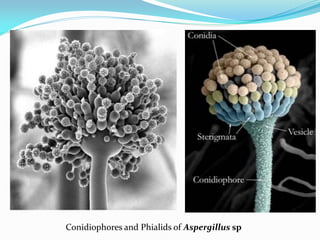
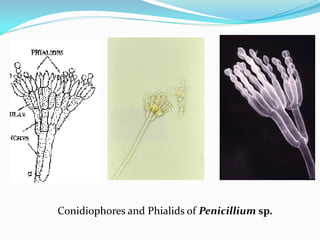
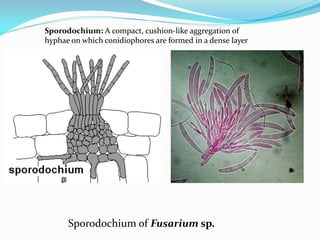
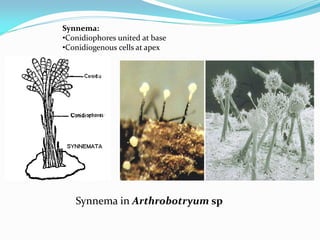
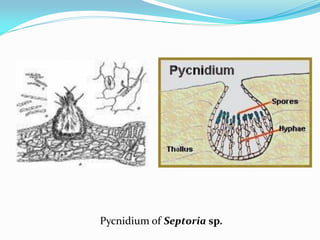
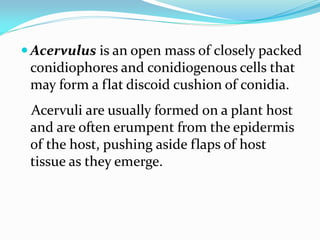
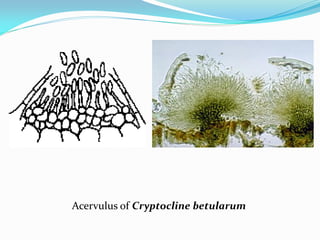
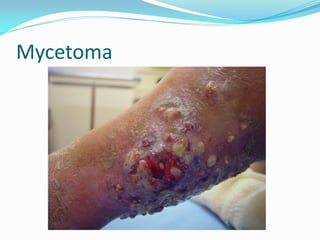
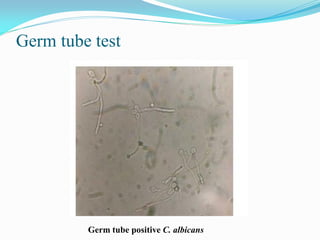
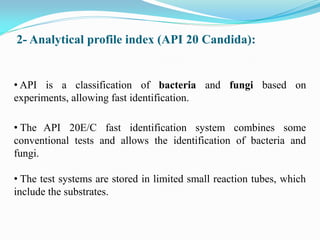
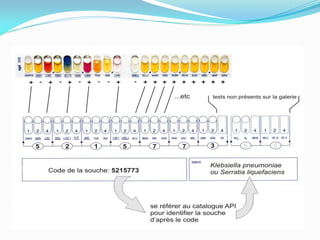
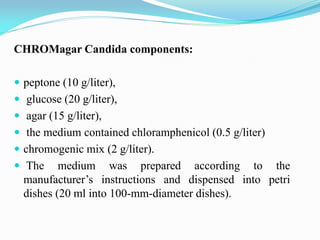
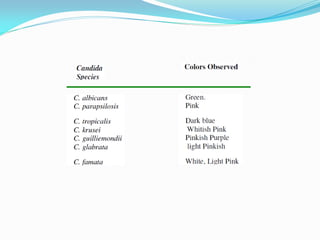
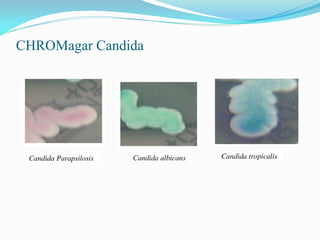
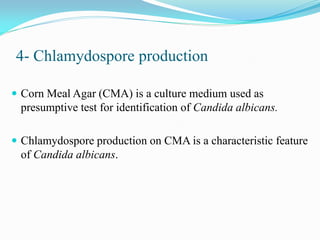
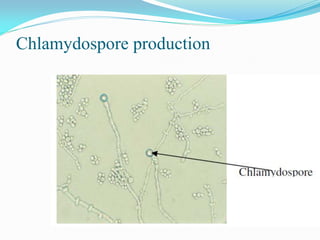
This document provides information about the phylum Ascomycota. It discusses key characteristics of yeasts and mycelial ascomycotina. Yeasts are unicellular fungi that can reproduce asexually through budding or fission, or sexually through formation of spores. Mycelial ascomycotina have septate mycelium and produce sexual spores (ascospores) within specialized structures called ascocarps, including cleistothecia, perithecia, apothecia, and ascostroma. The document describes the different types of ascocarps and ascus structures produced by ascomycetes.