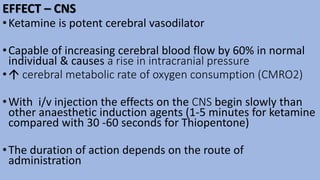
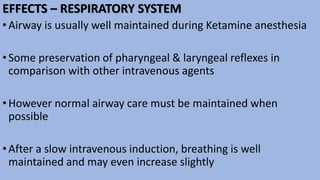
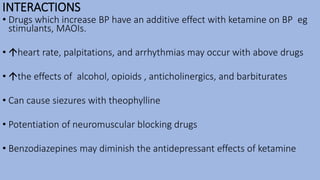
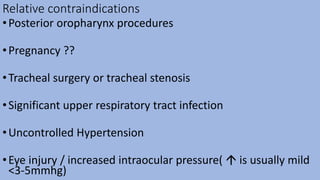
This document provides an overview of ketamine, including its history, pharmacology, effects, uses, and advantages for resource-poor settings. Some key points:
- Ketamine is a dissociative anesthetic and NMDA receptor antagonist first synthesized in 1962 and approved for use in 1970.
- It produces dissociative anesthesia while maintaining airway reflexes and cardiovascular stimulation.
- Ketamine has various uses including anesthesia, analgesia, and recently as a rapid-acting antidepressant at sub-anesthetic doses.
- Its safety profile and maintenance of airway reflexes make it advantageous for use in resource-poor settings without respiratory support.