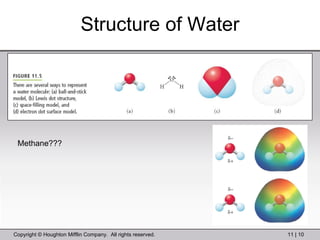
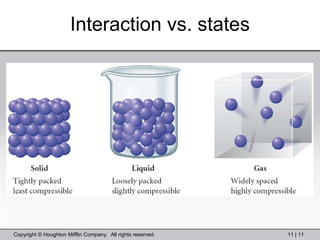
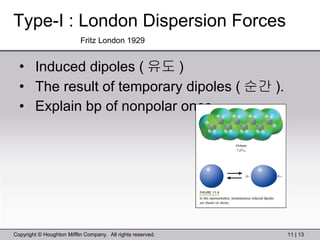
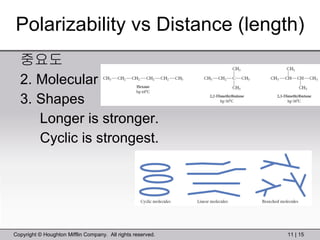
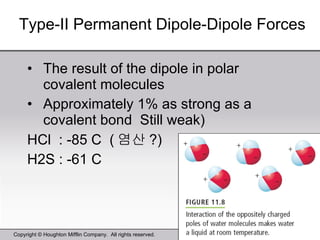
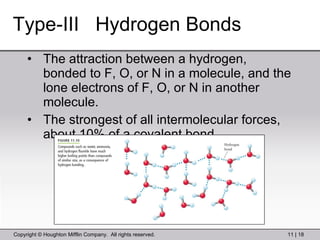
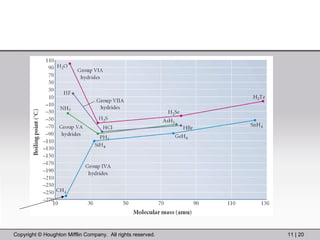
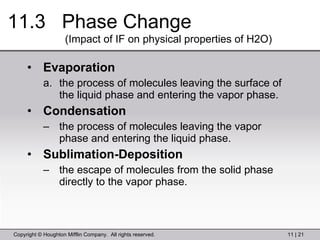
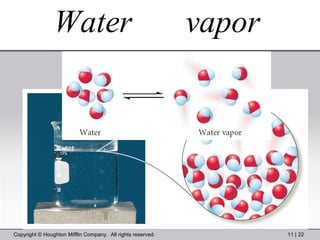
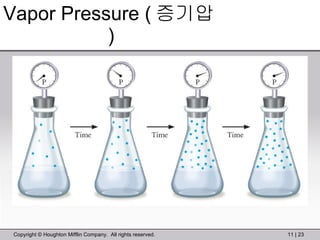
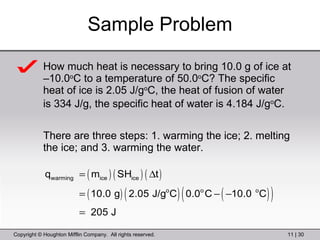
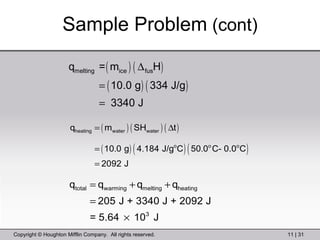
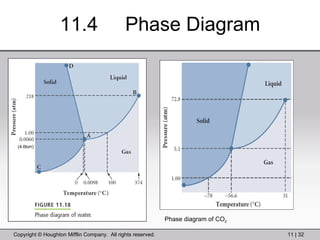
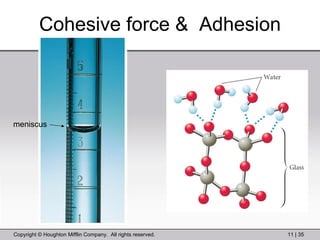
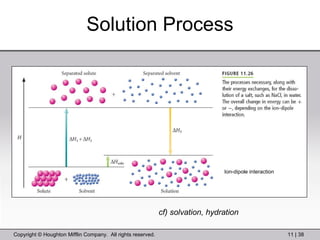
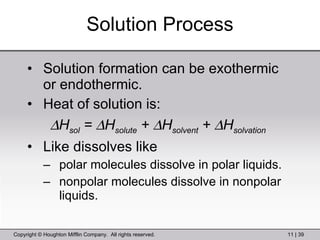
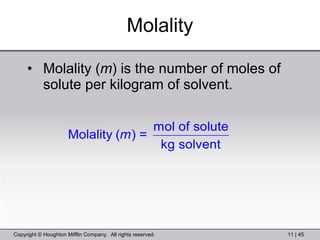
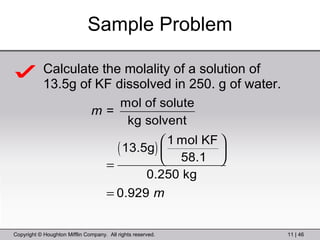
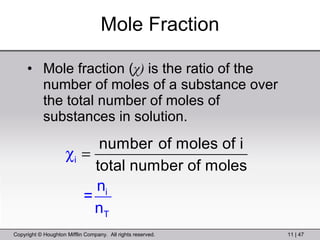
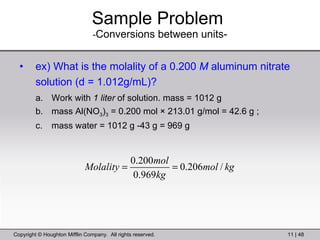
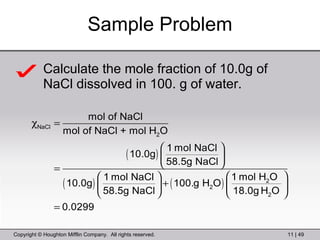
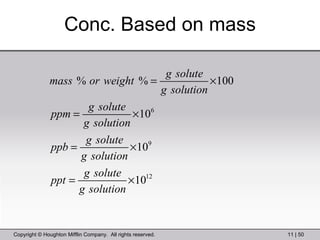
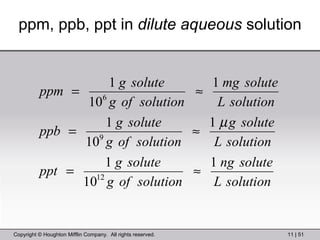
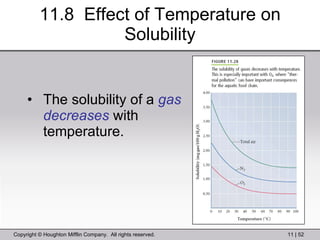
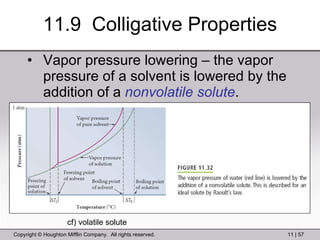
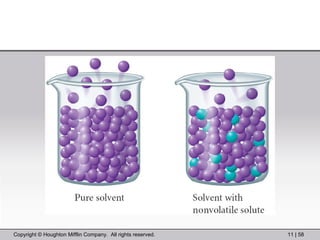
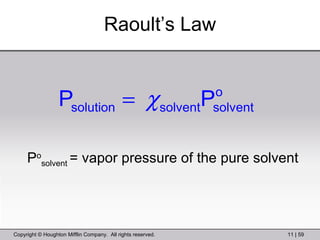
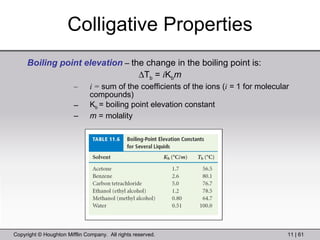
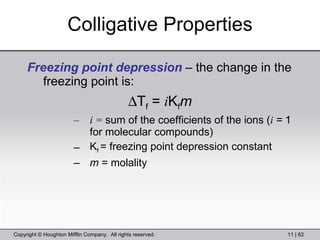
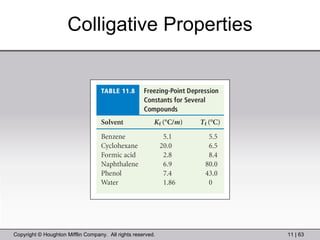
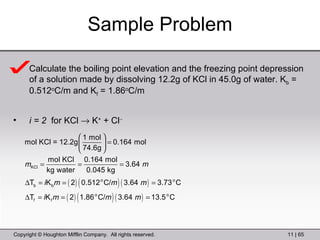
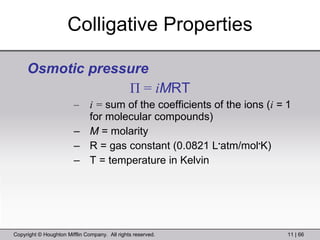
This document provides information about a General Chemistry 2 summer course including the instructor's contact information, teaching assistants, textbook, course schedule, exam details, and chapter outlines. Specifically, it outlines Chapter 11 which discusses the structure of water, intermolecular forces, physical properties of water and liquids, phase diagrams, solutions, and colligative properties.
![일반화학 2 (09 여름 ) 교수정보 : 이 동 수 ( 화학과 ) 2123-2641 [email_address] 과 442 조교정보 : 엄지원 , 채경수 연습문제 풀이 조교 : TBA](https://image.slidesharecdn.com/kelterch11-1296017078-phpapp02/75/kelter_ch11-1-2048.jpg)