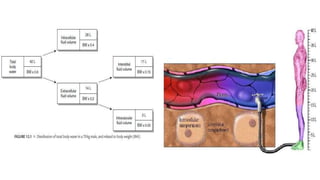
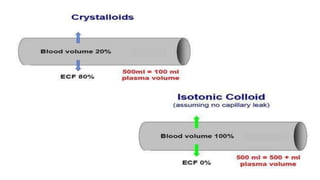
The document discusses fluid compartments and distribution in the human body, highlighting the composition of total body water, variations in fluid distribution among different demographics, and the principles governing fluid dynamics. It examines osmotic and oncotic pressure, Starling's principle, and the physiological mechanisms controlling fluid balance, including the effects of crystalloids and colloids in intravenous therapy. Additionally, it addresses the challenges in fluid management during clinical scenarios, emphasizing the importance of understanding fluid pharmacology for effective prescribing practices.













































![Jv = Kf ([Pc − Pi] − σ [πc − πsg])
Jv transcapillary flow
Kf filtration coefficient
Pc capillary hydrostatic pressure
Pi interstitial hydrostatic pressure
σ is the reflection coefficient
(the degree to which the tendency of a macromolecule
to cross the endothelial barrier is resisted)
πc capillary oncotic pressure
πsg subglycocalyx oncotic pressure](https://image.slidesharecdn.com/arunivf-200119013845/85/IV-FLUIDS-PART1-47-320.jpg)












































![STRONG ION DIFFERENCE (SID)
• It is the difference between strongest cation and strongest anion in a
particular compartment.
• Electrical neutrality needs cation = anions
• Strong ion difference + [H+] – [OH-] = 0
• Since hydroxyl ion is negligible ,
• Strong ion difference + [H+] = 0
• normal SID = Na – Cl
• = 140 – 103
• = 40 meq/ litre](https://image.slidesharecdn.com/arunivf-200119013845/85/IV-FLUIDS-PART1-93-320.jpg)
![• Strong ion difference + [H+] = 0
• therefore, if SID increases , [H+] decreases to maintain electrical neutrality.
• In 0.9% NaCl , SID=0
• Hence [H+] increases = pH decreases = acidosis.
• The SID of intravenous fluids determines their ability to influence the pH of
plasma. The SID of 0.9% NaCL is zero (Na – CL = 154 – 154 = 0) , so infusions
of 0.9% NaCL will reduce the SID of plasma and thereby reduce the plasma pH.
The SID of Ringer’s lactate fluid is 28 mEq/L (Na + K + Ca – CL= 130 + 4 + 3 –
109 = 28) if all the infused lactate is metabolized](https://image.slidesharecdn.com/arunivf-200119013845/85/IV-FLUIDS-PART1-94-320.jpg)






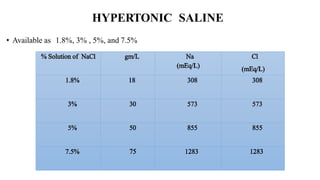

![2. Correction of hypoosmolar hyponatremia
3. Treatment of increased intracranial pressure
[Hypertonic saline may be superior to mannitol]
4. 7.5% - endothelial injury used as sclerosant](https://image.slidesharecdn.com/arunivf-200119013845/85/IV-FLUIDS-PART1-103-320.jpg)




![LACTATE BICARBONATE [ hepatic oxidation , gluconeogenesis]
ACETATE BICARBONATE, ACETOACETATE [ oxidised]
GLUCONATE GLUCOSE](https://image.slidesharecdn.com/arunivf-200119013845/85/IV-FLUIDS-PART1-108-320.jpg)

































































































