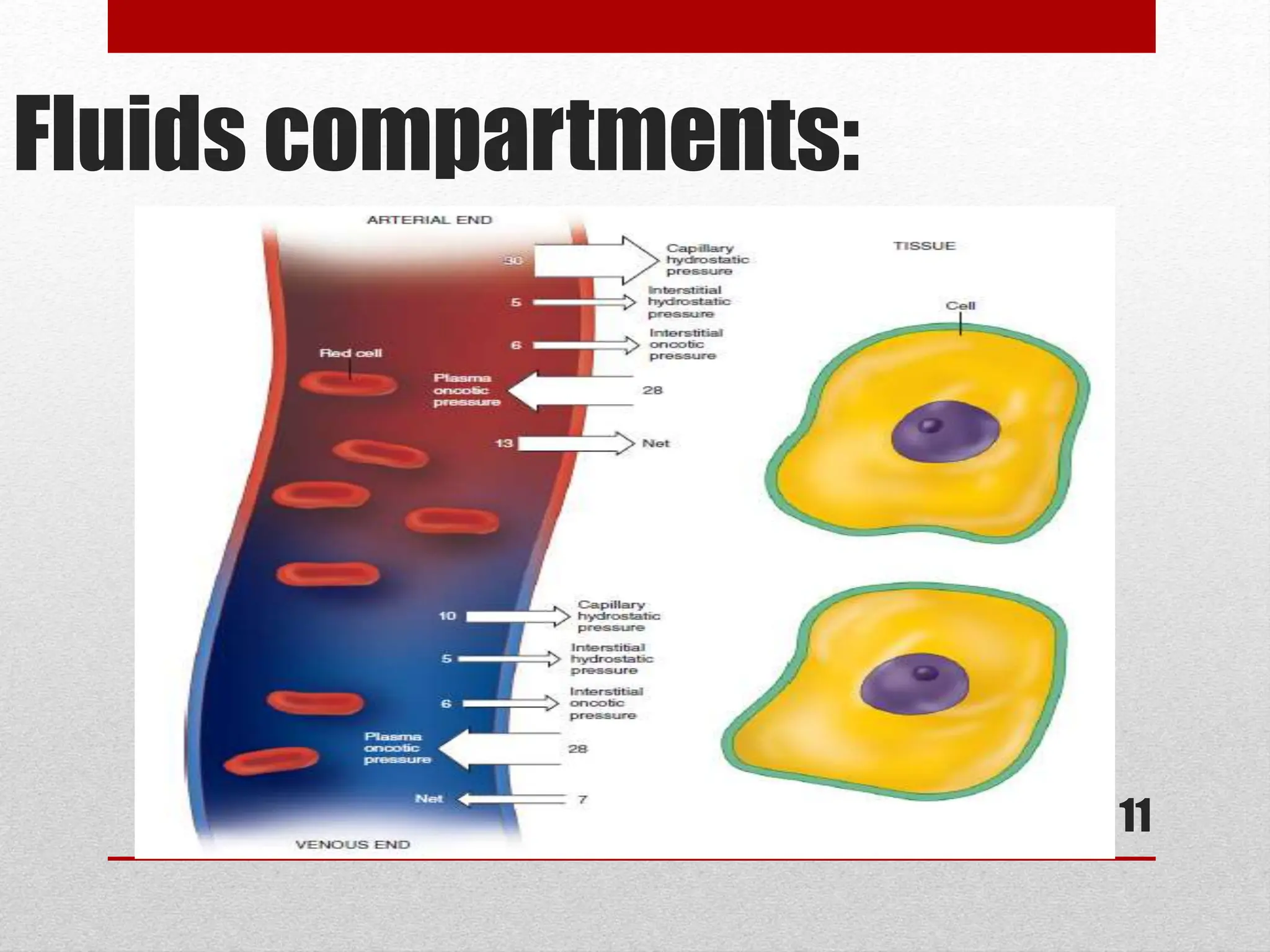
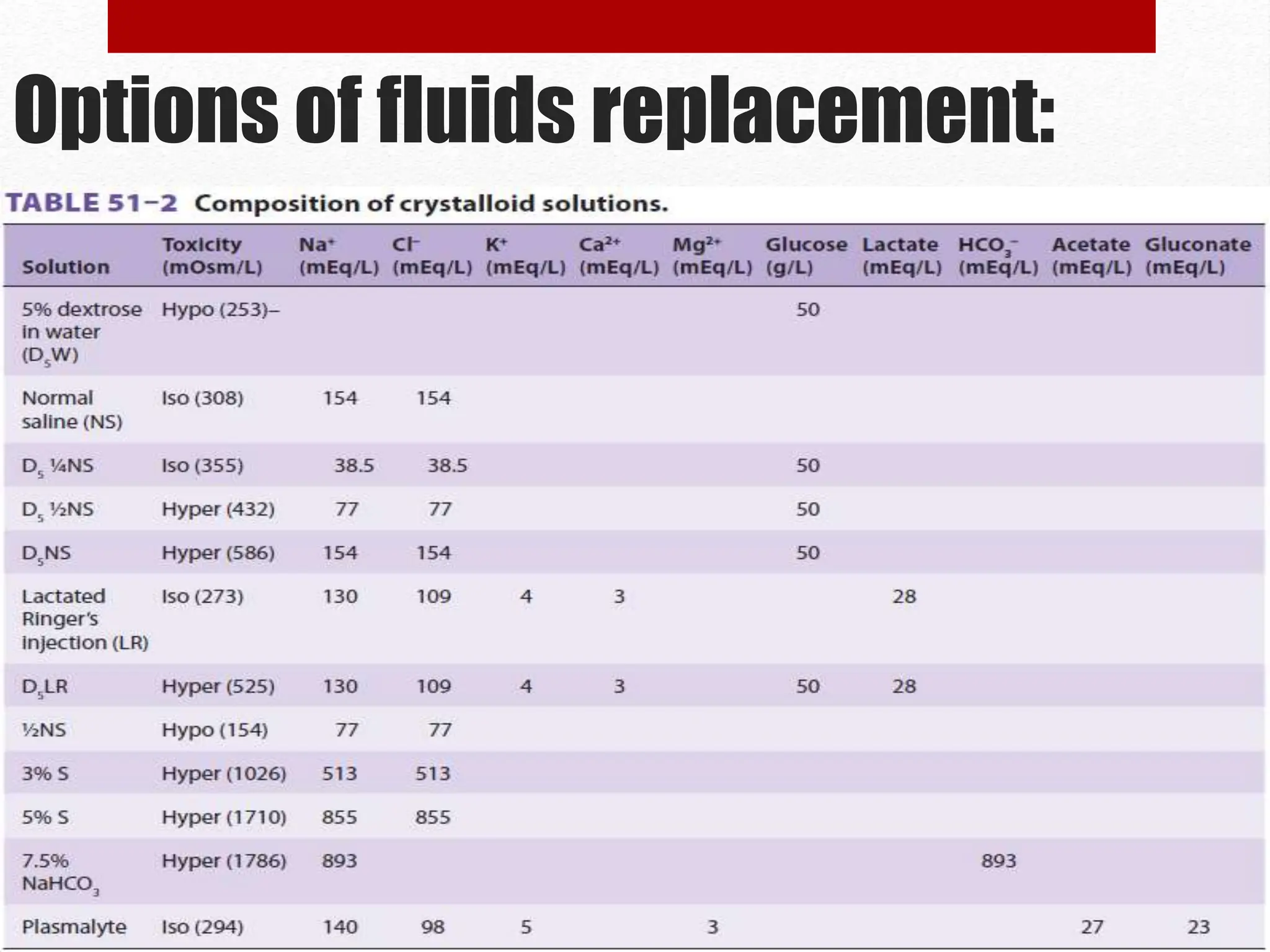
The document discusses the importance of intravenous fluid therapy during anesthesia, covering definitions, fluid compartments, and assessment of dehydrated patients. It details options for fluid replacement, including crystalloid and colloid solutions, along with methods for calculating fluid needs. Key factors in assessing fluid status and treatment options are also highlighted.